Final ID: 4361609
Loss of Adipose Lipophagy Impairs Lipid Mobilization and Exacerbates Obesity-Associated Metabolic Dysfunction
Abstract Body (Do not enter title and authors here): Introduction
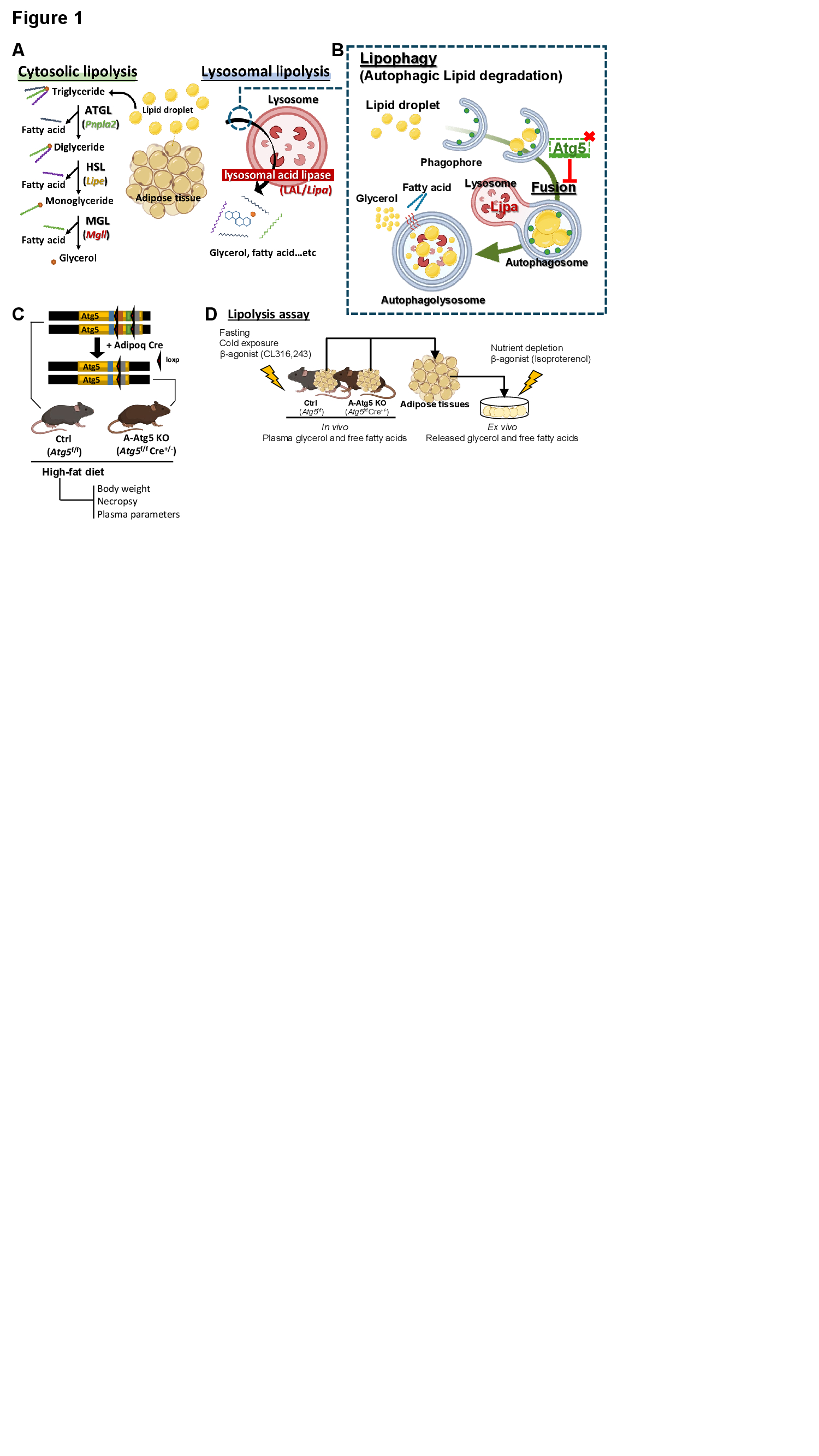
As of 2024, nearly 75% of U.S. adults were overweight or obese, conditions marked by overaccumulation of lipid in adipose tissue that impairs health. Obesity is a major risk factor for metabolic diseases, including diabetes and cardiovascular disease. Understanding lipid metabolism in obesity is critical for therapeutic development. Adipose tissue stores neutral lipids, mobilized via lipolysis during fasting or cold exposure. Canonical lipolysis, mediated by adipose triglyceride lipase (ATGL), releases free fatty acids (FFAs) and glycerol from triglycerides (Figure 1A). However, lipolysis persists in ATGL-deficient models, suggesting alternative mechanisms. We recently identified lysosomal lipolysis, mediated by lysosomal acid lipase (LAL), as a non-canonical lipid mobilization pathway in adipose tissue (Figure 1A). Yet, mechanisms regulating lipid trafficking to lysosomes remain unclear.
Hypothesis
We hypothesize that lipophagy, a selective form of autophagy, facilitates lysosomal lipolysis and plays a key role in adipocyte lipid metabolism (Figure 1B).
Methods
Public GEO datasets were analyzed to assess obesity-related changes in adipose gene expression. Adipose-specific Atg5 knockout (A-Atg5 KO) mice were fed a high-fat diet; metabolic parameters, glucose/insulin tolerance, and energy expenditure were measured (Figure 1C). In vivo lipolysis was assessed during fasting, cold exposure, and β-adrenergic stimulation (CL316,243) (Figure 1D). Ex vivo lipolysis was measured in explants from A-Atg5 KO mice under nutrient deprivation or CL treatment (Figure 1D).
Results
Autophagy genes and LAL were upregulated in obese adipose tissue. A-Atg5 KO mice showed no baseline defects but developed exacerbated obesity, glucose intolerance, insulin resistance, and reduced energy expenditure. Fasting-, cold-, and CL-induced elevations in plasma FFAs and glycerol were blunted, with impaired thermogenesis. Lipolysis was similarly reduced in adipose explants. Despite unchanged ATGL and LAL expression, ATGL inhibition further suppressed lipolysis in A-Atg5 KO adipocytes, revealing the complementary roles of cytosolic lipolysis and lipophagy.
Conclusion
Lipophagy is a critical regulator of adipocyte lipid mobilization, working in concert with cytosolic lipolysis under metabolic stress. These findings link adipose lipophagy to obesity and metabolic dysfunction, highlighting it as a potential therapeutic target.
As of 2024, nearly 75% of U.S. adults were overweight or obese, conditions marked by overaccumulation of lipid in adipose tissue that impairs health. Obesity is a major risk factor for metabolic diseases, including diabetes and cardiovascular disease. Understanding lipid metabolism in obesity is critical for therapeutic development. Adipose tissue stores neutral lipids, mobilized via lipolysis during fasting or cold exposure. Canonical lipolysis, mediated by adipose triglyceride lipase (ATGL), releases free fatty acids (FFAs) and glycerol from triglycerides (Figure 1A). However, lipolysis persists in ATGL-deficient models, suggesting alternative mechanisms. We recently identified lysosomal lipolysis, mediated by lysosomal acid lipase (LAL), as a non-canonical lipid mobilization pathway in adipose tissue (Figure 1A). Yet, mechanisms regulating lipid trafficking to lysosomes remain unclear.
Hypothesis
We hypothesize that lipophagy, a selective form of autophagy, facilitates lysosomal lipolysis and plays a key role in adipocyte lipid metabolism (Figure 1B).
Methods
Public GEO datasets were analyzed to assess obesity-related changes in adipose gene expression. Adipose-specific Atg5 knockout (A-Atg5 KO) mice were fed a high-fat diet; metabolic parameters, glucose/insulin tolerance, and energy expenditure were measured (Figure 1C). In vivo lipolysis was assessed during fasting, cold exposure, and β-adrenergic stimulation (CL316,243) (Figure 1D). Ex vivo lipolysis was measured in explants from A-Atg5 KO mice under nutrient deprivation or CL treatment (Figure 1D).
Results
Autophagy genes and LAL were upregulated in obese adipose tissue. A-Atg5 KO mice showed no baseline defects but developed exacerbated obesity, glucose intolerance, insulin resistance, and reduced energy expenditure. Fasting-, cold-, and CL-induced elevations in plasma FFAs and glycerol were blunted, with impaired thermogenesis. Lipolysis was similarly reduced in adipose explants. Despite unchanged ATGL and LAL expression, ATGL inhibition further suppressed lipolysis in A-Atg5 KO adipocytes, revealing the complementary roles of cytosolic lipolysis and lipophagy.
Conclusion
Lipophagy is a critical regulator of adipocyte lipid mobilization, working in concert with cytosolic lipolysis under metabolic stress. These findings link adipose lipophagy to obesity and metabolic dysfunction, highlighting it as a potential therapeutic target.
More abstracts on this topic:
Adiposomal microRNAs Mediate Vascular Dysfunction in Obesity-Associated Type 2 Diabetes
Mirza Imaduddin, Morsy Mohammed, Levitan Irena, Raj Usha, Mahmoud Abeer
3-Mercaptopyruvate Sulfurtransferase is a Critical Regulator of Branched-Chain Amino Acid Catabolism in Cardiometabolic HFpEFLi Zhen, Doiron Jake, Xia Huijing, Lapenna Kyle, Sharp Thomas, Yu Xiaoman, Nagahara Noriyuki, Goodchild Traci, Lefer David