Final ID: MP1723
Effect Of Inclisiran-based Treatment Strategy, In Combination With Individually Optimized Statin Therapy, On Quality Of Life And Muscle-related Pain vs. Standard of Care: Exploratory Outcomes From The VICTORION-Difference Study
Abstract Body (Do not enter title and authors here): Background: Inclisiran provides sustained and effective LDL-C reduction in individuals with hyperlipidemia with a favorable long-term safety profile; however, its impact on QoL remains unknown. The VICTORION-Difference study evaluated the early efficacy, safety, as well as QoL outcomes with inclisiran vs. placebo on top of individually optimized lipid-lowering therapy (ioLLT; standard of care [SOC]) in individuals with hypercholesterolemia who have not achieved guideline-recommended LDL-C goals.
Research question: Whether inclisiran-based treatment strategy vs. SOC reduces muscle-related adverse events (MRAEs) and days with pain experienced. Data on additional key secondary and exploratory endpoints for efficacy and safety, including LDL-C reduction, pain-related QoL scores, AEs and serious AEs will be presented.
Methods: This phase 4, double-blind, placebo-controlled trial included adults with hypercholesterolemia at high or very high CV risk on maximally tolerated dose (MTD) of statins. Participants were randomized 1:1 to receive subcutaneous injections of 300 mg inclisiran sodium or placebo on top of SOC at Days 1, 90, and 270. At randomization, participants received open-label rosuvastatin as background ioLLT; starting dose of 5 mg/day or 10 mg/day and sequentially titrated to the MTD. A logistic regression model analyzed the proportion of participants experiencing ≥1 MRAE from Day 1 to Day 360, while a negative binomial model assessed the annualized number of pain days. The study was powered for the key secondary endpoint related to MRAEs.
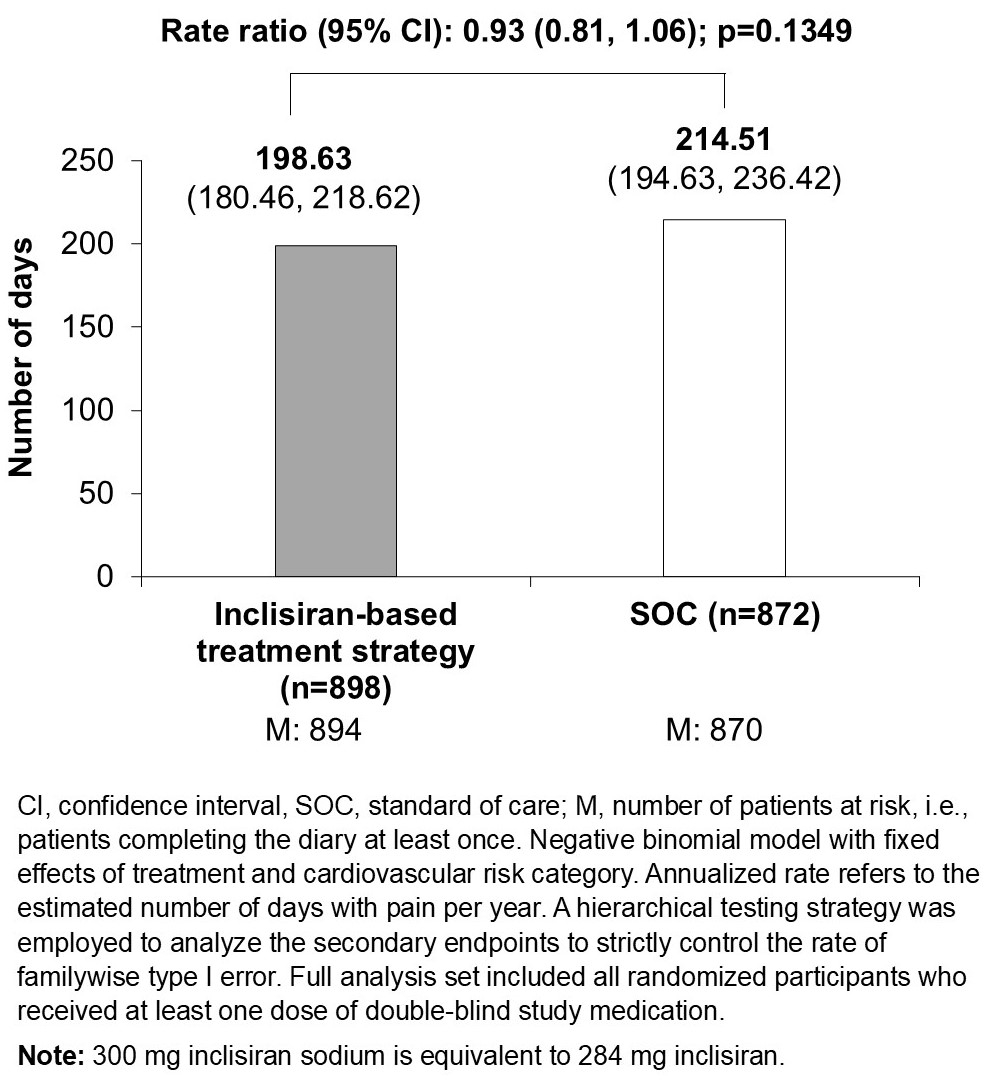
Results: A total of 1770 individuals (mean age, 63.7 years) were randomized to receive study medication (inclisiran, n=898; SOC, n=872), with 92.3% of participants classified as having very high CV risk. Fewer participants in the inclisiran vs. SOC arm experienced a MRAE (11.9% vs. 19.2%; odds ratio=0.57; 95% CI: 0.43, 0.74). Participants receiving inclisiran experienced numerically fewer days with pain vs. SOC (198.63 vs. 214.51; rate ratio: 0.93; 95% CI: 0.81, 1.06; Figure).
Conclusion: VICTORION-Difference is the largest LDL-C–lowering study from the inclisiran clinical development program so far, and the first to evaluate the effect of inclisiran vs. placebo on top of SOC using patient-centric QoL scores as secondary endpoints. Overall, inclisiran-based treatment strategy vs. SOC led to significantly fewer MRAEs and a numerical reduction in the number of days individuals with hypercholesterolemia experienced pain.
Research question: Whether inclisiran-based treatment strategy vs. SOC reduces muscle-related adverse events (MRAEs) and days with pain experienced. Data on additional key secondary and exploratory endpoints for efficacy and safety, including LDL-C reduction, pain-related QoL scores, AEs and serious AEs will be presented.
Methods: This phase 4, double-blind, placebo-controlled trial included adults with hypercholesterolemia at high or very high CV risk on maximally tolerated dose (MTD) of statins. Participants were randomized 1:1 to receive subcutaneous injections of 300 mg inclisiran sodium or placebo on top of SOC at Days 1, 90, and 270. At randomization, participants received open-label rosuvastatin as background ioLLT; starting dose of 5 mg/day or 10 mg/day and sequentially titrated to the MTD. A logistic regression model analyzed the proportion of participants experiencing ≥1 MRAE from Day 1 to Day 360, while a negative binomial model assessed the annualized number of pain days. The study was powered for the key secondary endpoint related to MRAEs.
Results: A total of 1770 individuals (mean age, 63.7 years) were randomized to receive study medication (inclisiran, n=898; SOC, n=872), with 92.3% of participants classified as having very high CV risk. Fewer participants in the inclisiran vs. SOC arm experienced a MRAE (11.9% vs. 19.2%; odds ratio=0.57; 95% CI: 0.43, 0.74). Participants receiving inclisiran experienced numerically fewer days with pain vs. SOC (198.63 vs. 214.51; rate ratio: 0.93; 95% CI: 0.81, 1.06; Figure).
Conclusion: VICTORION-Difference is the largest LDL-C–lowering study from the inclisiran clinical development program so far, and the first to evaluate the effect of inclisiran vs. placebo on top of SOC using patient-centric QoL scores as secondary endpoints. Overall, inclisiran-based treatment strategy vs. SOC led to significantly fewer MRAEs and a numerical reduction in the number of days individuals with hypercholesterolemia experienced pain.
More abstracts on this topic:
A hepatic steatosis-mediated metabolite reprograms macrophage lipid metabolism and aggravates atherosclerosis
Long Ting, Feng Ruijia, Feng Weiqi, Peng Guiyan, Yang Wenchao, Li Zilun, Huang Kan, Chang Guangqi
Cardiovascular Stroke Nursing Best Abstract Award: Digital Health-Based Interventions Improve Healthy Behaviors, Weight Loss, and Psychological Well-Being in Older Adults at Risk for Cardiovascular DiseasCandelaria Dion, Reyes Andrew Thomas, Serafica Reimund, Hildebrand Janett, Cacciata Marysol, Sta. Maria Axel, Lee Jung-ah, Stromberg Anna, Evangelista Lorraine