Final ID: MP947
Transcriptomic profiling of plasma microRNA reveals circulating biomarkers of transthyretin cardiac amyloidosis and dysregulated signaling pathways.
Abstract Body (Do not enter title and authors here): Background: Early diagnosis and initiation of disease modifying therapy for transthyretin amyloid cardiomyopathy (ATTR-CM) improves prognosis. However, ATTR-CM remains underdiagnosed and can be commonly mistaken for hypertensive left ventricular hypertrophy (hLVH). Plasma transcriptomic profiling may help distinguish ATTR-CM from hLVH and provide insights on its pathophysiology.
Hypothesis: Circulating microRNAs can distinguish ATTR-CM from hLVH and identify dysregulated signaling pathways.
Methods: In this case-control study, we sequenced plasma microRNAs in cases with ATTR-CM and age- and sex-matched controls with hLVH. We developed a microRNA-based Lasso model to discriminate cases from controls in a training set consisting of the earliest enrolled 2/3 of the cohort. We defined the later enrolled 1/3 as the prospective test set for validation. We screened candidate microRNAs for model training based on their independent associations with ATTR-CM after adjustment for clinical characteristics that differed between the 2 groups. We compared the performance of the microRNA model in the test set to the Mayo ATTR-CM risk score using DeLong’s test and net reclassification improvement (NRI). Separately, we identified microRNAs that were differentially expressed between cases and controls (Bonferroni-adjusted P <0.05) and performed a pathway analysis of these microRNAs. We considered a pathway as dysregulated if Benjamini–Hochberg false discovery rate was <0.05 and at least 2 pathway-associated microRNAs were over- or under-represented in ATTR-CM cases.
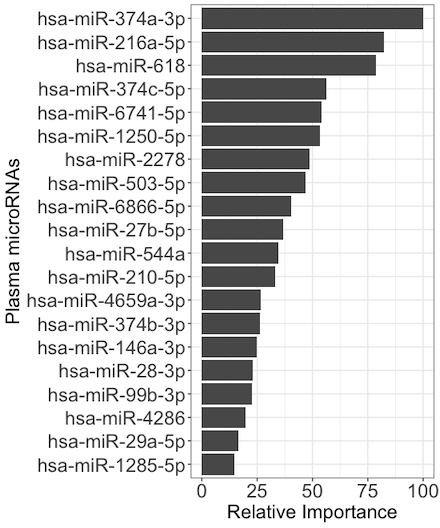
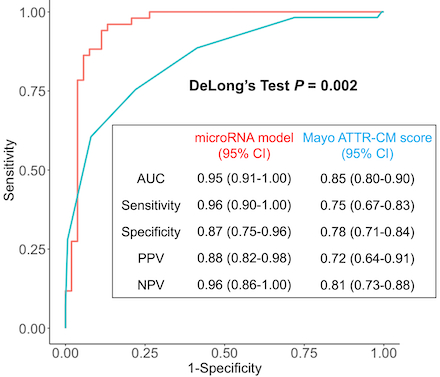
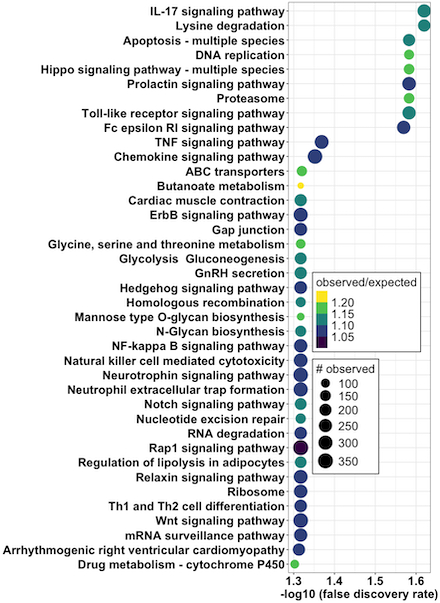
Results: A total of 311 participants (152 ATTR-CA, 159 LVH) were included. Of the 917 sequenced microRNAs, 48 were independently associated with ATTR-CM and used for model development in the training set. The top 20 most important microRNAs are shown in Figure 1. The microRNA model had an area under the receiver-operating-characteristic curve (AUC) of 0.95 (95% CI, 0.91-1.00) in the test set, outperforming the Mayo ATTR-CM score’s AUC of 0.85 (95% CI, 0.80-0.90; P = 0.002, Figure 2). In the test set, compared to the Mayo ATTR-CM score, the microRNA model had a positive NRI (0.21, 98% CI 0.008-0.41). Pathway analysis revealed 39 dysregulated pathways in patients with ATTR-CM, including the Hippo and tumor necrosis factor pathways (Figure 3).
Conclusions: This study identified circulating microRNAs that distinguished ATTR-CM from hLVH with high accuracy and identified signaling pathways associated with ATTR-CM.
Hypothesis: Circulating microRNAs can distinguish ATTR-CM from hLVH and identify dysregulated signaling pathways.
Methods: In this case-control study, we sequenced plasma microRNAs in cases with ATTR-CM and age- and sex-matched controls with hLVH. We developed a microRNA-based Lasso model to discriminate cases from controls in a training set consisting of the earliest enrolled 2/3 of the cohort. We defined the later enrolled 1/3 as the prospective test set for validation. We screened candidate microRNAs for model training based on their independent associations with ATTR-CM after adjustment for clinical characteristics that differed between the 2 groups. We compared the performance of the microRNA model in the test set to the Mayo ATTR-CM risk score using DeLong’s test and net reclassification improvement (NRI). Separately, we identified microRNAs that were differentially expressed between cases and controls (Bonferroni-adjusted P <0.05) and performed a pathway analysis of these microRNAs. We considered a pathway as dysregulated if Benjamini–Hochberg false discovery rate was <0.05 and at least 2 pathway-associated microRNAs were over- or under-represented in ATTR-CM cases.
Results: A total of 311 participants (152 ATTR-CA, 159 LVH) were included. Of the 917 sequenced microRNAs, 48 were independently associated with ATTR-CM and used for model development in the training set. The top 20 most important microRNAs are shown in Figure 1. The microRNA model had an area under the receiver-operating-characteristic curve (AUC) of 0.95 (95% CI, 0.91-1.00) in the test set, outperforming the Mayo ATTR-CM score’s AUC of 0.85 (95% CI, 0.80-0.90; P = 0.002, Figure 2). In the test set, compared to the Mayo ATTR-CM score, the microRNA model had a positive NRI (0.21, 98% CI 0.008-0.41). Pathway analysis revealed 39 dysregulated pathways in patients with ATTR-CM, including the Hippo and tumor necrosis factor pathways (Figure 3).
Conclusions: This study identified circulating microRNAs that distinguished ATTR-CM from hLVH with high accuracy and identified signaling pathways associated with ATTR-CM.
More abstracts on this topic:
A Case of Recurrent Acute Coronary Syndrome and Cardiogenic Shock due to Apolipoprotein A-IV Amyloidosis
Muthukkumar Rashmi, Holmes Taylor, Friede Kevin
A First-in-Class EV-miRNA Diagnostic System for Early Identification of IVIG-Resistant Kawasaki DiseaseNakaoka Hideyuki, Hirono Keiichi, Hara Akane, Tsuboi Kaori, Ibuki Keijiro, Ozawa Sayaka, Ichida Fukiko