Final ID: MP1028
Lipopolysaccharide-induced Tolerance Educates Hematopoietic Stem Cells to Protect against Cardiac Allografts Rejection
Abstract Body (Do not enter title and authors here): Backgrounds: Heart transplantation remains the gold-standard therapy for end-stage heart failure. However, immune rejection of the transplanted heart critically compromises long-term survival rates. Accumulating evidence suggests that the contribution of Lipopolysaccharide (LPS)-induced innate immunity to immune rejection has been historically underappreciated, which may partially explain the limited efficacy of current immunosuppressive regimens in clinical practice.
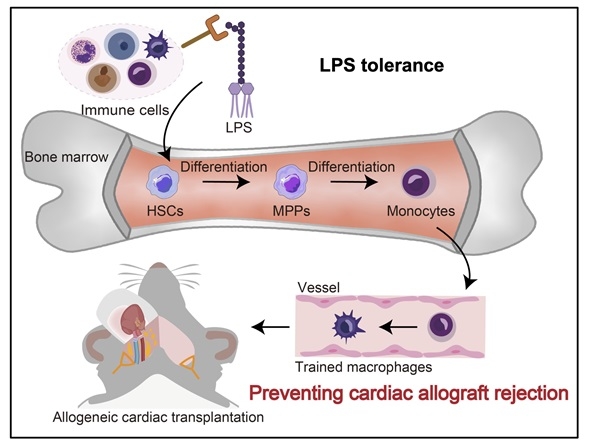
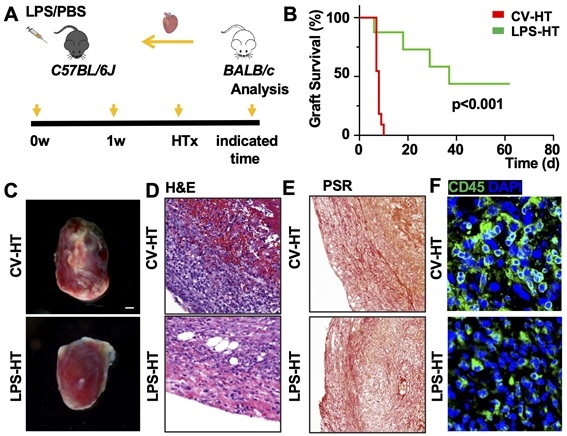
Methods: Using a novel mouse model that combines LPS-tolerance with heart transplantation, we systematically explore how LPS-tolerance modulates the immune microenvironment of transplanted hearts through epigenetic remodeling and metabolic reprogramming. By transferring whole bone marrow from CAG-Cre; R26-tdTomato mice to irradiated C57BL/6J mice, chimeric mice were generated. Subsequently, C57BL/6J mice received LPS-educated hematopoietic stem cells (HSCs) before undergoing heart transplantation.
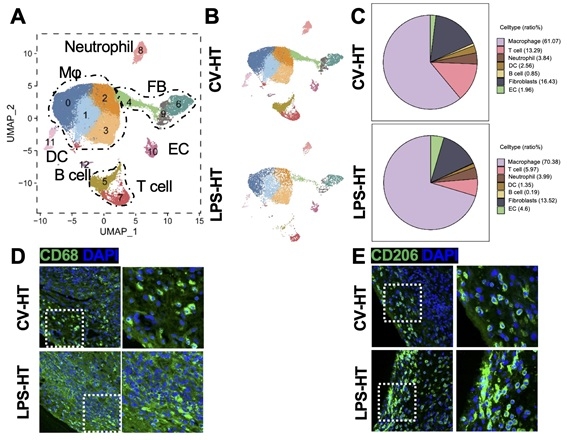
Results: Here, we showed that LPS exposure altered the transcriptome of HSCs and induced myelopoiesis. Preliminary findings showed LPS-educated HSCs generate epigenetically modified macrophages that provided significantly better protection against cardiac allografts rejection. An increase in CD206+ M2 macrophages were identified in LPS-treated allografts associated with the presence of the epigenetic marker H3K27ac, indicating active gene expression. Furthermore, by integrating the established heart transplantation biobank with comprehensive multi-omics analyses, such as transcriptomics and epigenomics, we aimed to identify distinct molecular signatures associated with this LPS tolerance-driven graft protection. The novel therapeutic targets for attenuating immune rejection via trained immunity were identified and their clinical translational potential being validated.
Conclusions: Distinct from conventional immunosuppressive agents targeting T cell-mediated adaptive immunity, we pioneer a paradigm shift by investigating the role of LPS-induced innate immunity in cardiac allograft rejection. The clinical translation of these findings is anticipated to revolutionize immunosuppressive strategies, ultimately benefiting a substantial population of heart failure patients.
Methods: Using a novel mouse model that combines LPS-tolerance with heart transplantation, we systematically explore how LPS-tolerance modulates the immune microenvironment of transplanted hearts through epigenetic remodeling and metabolic reprogramming. By transferring whole bone marrow from CAG-Cre; R26-tdTomato mice to irradiated C57BL/6J mice, chimeric mice were generated. Subsequently, C57BL/6J mice received LPS-educated hematopoietic stem cells (HSCs) before undergoing heart transplantation.
Results: Here, we showed that LPS exposure altered the transcriptome of HSCs and induced myelopoiesis. Preliminary findings showed LPS-educated HSCs generate epigenetically modified macrophages that provided significantly better protection against cardiac allografts rejection. An increase in CD206+ M2 macrophages were identified in LPS-treated allografts associated with the presence of the epigenetic marker H3K27ac, indicating active gene expression. Furthermore, by integrating the established heart transplantation biobank with comprehensive multi-omics analyses, such as transcriptomics and epigenomics, we aimed to identify distinct molecular signatures associated with this LPS tolerance-driven graft protection. The novel therapeutic targets for attenuating immune rejection via trained immunity were identified and their clinical translational potential being validated.
Conclusions: Distinct from conventional immunosuppressive agents targeting T cell-mediated adaptive immunity, we pioneer a paradigm shift by investigating the role of LPS-induced innate immunity in cardiac allograft rejection. The clinical translation of these findings is anticipated to revolutionize immunosuppressive strategies, ultimately benefiting a substantial population of heart failure patients.
More abstracts on this topic:
Aberrant Regulation of endMT in Turner Syndrome: Implications for the Pathogenesis of Congenital Cardiovascular Disease
Garcia Huitron Eric Ivan, Zhang Xiaoying, Babcock Lance, Grande-allen Kathryn, Prakash Siddharth
Cardiovascular Effectiveness of Glucagon-like Peptide-1 Receptor Agonists and Empagliflozin Combination Therapy in Adults with Type 2 DiabetesHtoo Phyo, Patorno Elisabetta, Paik Julie, Tesfaye Helen, Schneeweiss Sebastian, Glynn Robert, Shay Christina, Schmedt Niklas, Koeneman Lisette, Wexler Deborah