Final ID: Mo1063
CD34+ Cells Contributed to Cardiac Fibrosis Are Regulated by Hyperlipidemia
Abstract Body (Do not enter title and authors here): Background: Lipid metabolic alterations are emerging as significant mechanisms in the prognosis of cardiac remodeling induced by hypertension and could impact the therapies for cardiac fibrosis. The fatty acid transport/oxidation gene FABP4 acts as a metabolic and inflammation regulator, whose inhibition can attenuate kidney and renal interstitial fibrosis. However, its role in the heart remains unclear. Our study aimed to evaluate the density of FABP4 in patients and mouse model, and its association with cardiac fibrosis.
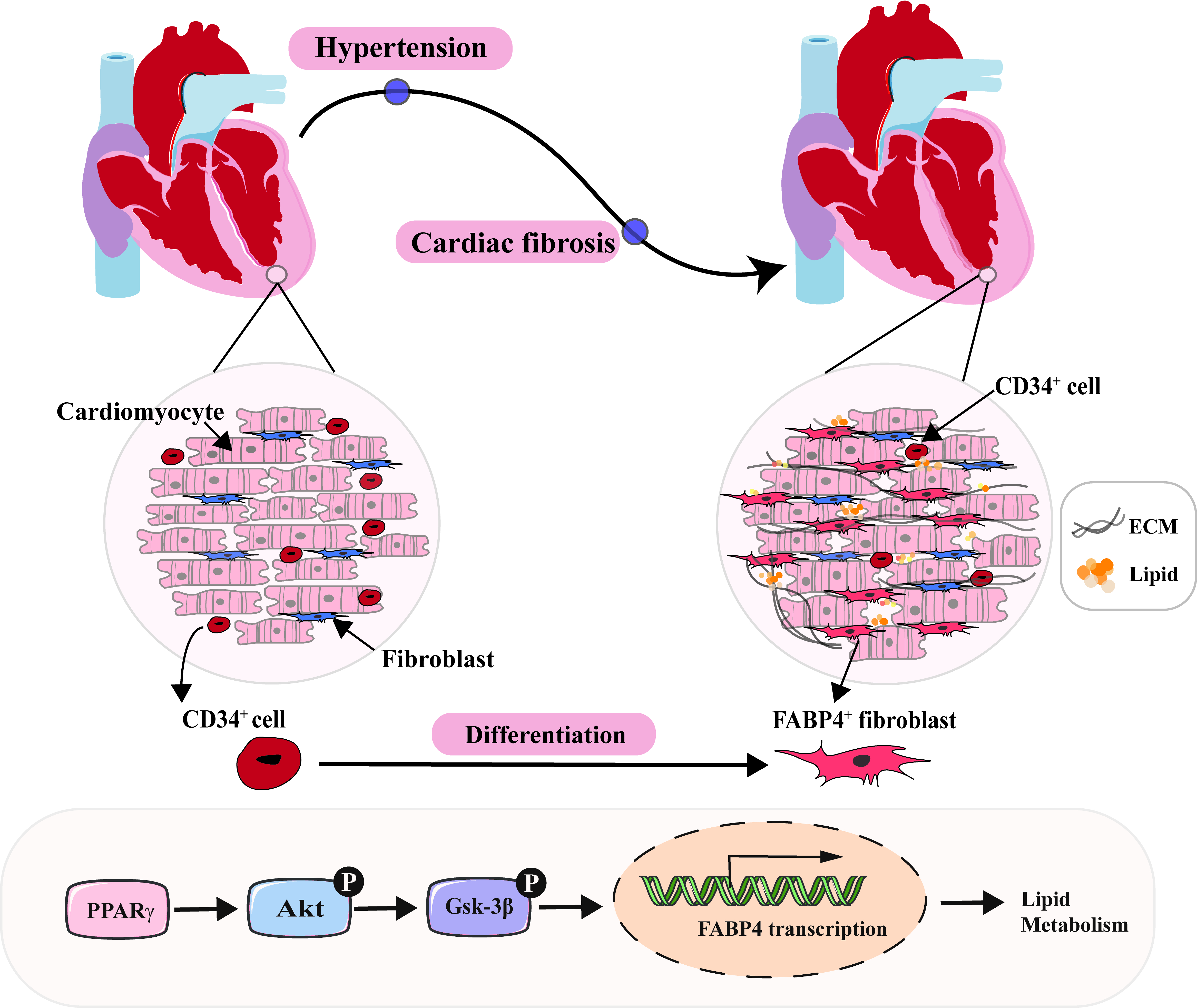
Methods and Results: Single cell RNA sequencing of the heart and enriched Cd34-tdTomato+ lineage cells in human and a murine model treated with angiotensin II were performed. Through the analysis of single-cell transcriptomes extracted from mouse hearts and human hearts tissues, we explored the transcriptomic landscape throughout cardiac fibrosis and revealed dysregulation of cardiac fibrosis, fatty acid transport/oxidation, and PPARγ signaling in the failing heart. We identified in which the FABP4+ fibroblasts were increased in diseased tissues accompanied by the accumulation of lipid droplets and cardiac fibrosis. Using pseudotime analysis, inducible lineage tracing and bone marrow transplantation models, our study showed that hypertension can expedite the differentiation of non-bone marrow-derived CD34+ cells into FABP4+ fibroblasts, leading to lipid accumulation and cardiac fibrosis. Furthermore, the PPARγ/Akt/Gsk3β pathway plays a critical role in orchestrating the differentiation process. Interestingly, both in vitro and in vivo, partial depletion of CD34+ cells using diphtheria toxin or pioglitazone treatment resulted in a notable reduction of FABP4+ fibroblasts and triglyceride in heart, along with an improvement in cardiac function through the PPARγ pathway. Additionally, GW9662, a selective antagonist of PPARγ, markedly reduced cell lipotoxicity and cardiac fibrosis.
Conclusions: Collectively, our study provides valuable insights into the cellular landscape of the fibrotic heart with hypertension, indicating that the differentiation of non-bone marrow-derived CD34+ cells into FABP4+ fibroblasts accelerates the lipid accumulation and progression of cardiac fibrosis via PPARγ pathway.
Methods and Results: Single cell RNA sequencing of the heart and enriched Cd34-tdTomato+ lineage cells in human and a murine model treated with angiotensin II were performed. Through the analysis of single-cell transcriptomes extracted from mouse hearts and human hearts tissues, we explored the transcriptomic landscape throughout cardiac fibrosis and revealed dysregulation of cardiac fibrosis, fatty acid transport/oxidation, and PPARγ signaling in the failing heart. We identified in which the FABP4+ fibroblasts were increased in diseased tissues accompanied by the accumulation of lipid droplets and cardiac fibrosis. Using pseudotime analysis, inducible lineage tracing and bone marrow transplantation models, our study showed that hypertension can expedite the differentiation of non-bone marrow-derived CD34+ cells into FABP4+ fibroblasts, leading to lipid accumulation and cardiac fibrosis. Furthermore, the PPARγ/Akt/Gsk3β pathway plays a critical role in orchestrating the differentiation process. Interestingly, both in vitro and in vivo, partial depletion of CD34+ cells using diphtheria toxin or pioglitazone treatment resulted in a notable reduction of FABP4+ fibroblasts and triglyceride in heart, along with an improvement in cardiac function through the PPARγ pathway. Additionally, GW9662, a selective antagonist of PPARγ, markedly reduced cell lipotoxicity and cardiac fibrosis.
Conclusions: Collectively, our study provides valuable insights into the cellular landscape of the fibrotic heart with hypertension, indicating that the differentiation of non-bone marrow-derived CD34+ cells into FABP4+ fibroblasts accelerates the lipid accumulation and progression of cardiac fibrosis via PPARγ pathway.
More abstracts on this topic:
Adipocyte Enhancer Binding Protein 1 (AEBP1) Inhibition as a Potential Anti-Fibrotic Therapy in Heart Failure
AAV-mediated Gene Delivery of PERM1 Prevents the Development of Heart Failure with Reduced Ejection Fraction in a Mouse Model of Pressure Overload
Shankar Thirupura S, Calder Dallen, Sachse Frank, Kyriakopoulos Christos, Maneta Eleni, Srinivasan Harini, Tseliou Eleni, Navankasattusas Sutip, Selzman Craig, Boudina Sihem, Seidel Thomas, Visker Joseph, Lavine Kory, Drakos Stavros, Amrute Junedh, Polishchuk Georgiy, Lunde J Ty, Ling Jing, Ferrin Peter, Hamouche Rana, Feigle Dominik
AAV-mediated Gene Delivery of PERM1 Prevents the Development of Heart Failure with Reduced Ejection Fraction in a Mouse Model of Pressure Overload
Sreedevi Karthi, Doku Abigail Oforiwaa, Thomas Rebekah, Salama Sarah, Zaitsev Alexey, Warren Junco