Final ID: P2146
Multiple Pathways of CD34+ Cell Differentiation during Embryogenesis
Abstract Body: Introduction: CD34+ progenitor cells are widely used for stem cell therapy for cardiovascular diseases, but the effectiveness of the treatment is variable. To understand the fundamental mechanism of CD34+ cell development, we aimed to investigate cell fates of CD34+ progenitors during the embryogenesis of mice and humans.
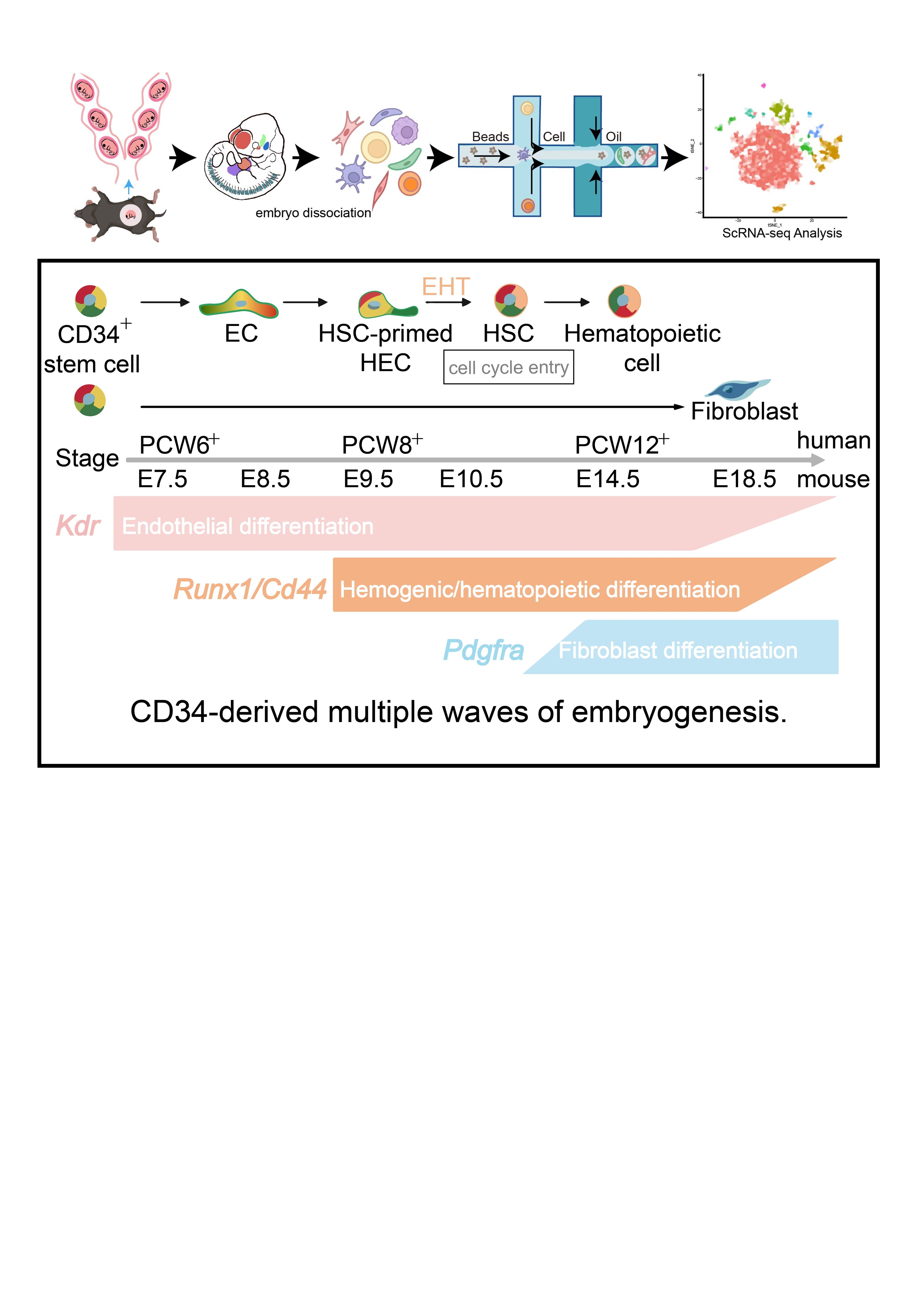
Methods: Human embryos from PCW 6 to 12 were obtained from patients who had undergone induced or spontaneous abortions and samples were subjected to mass spectrometry. For the animal model, embryos were obtained among C57BL/6J, Cd34-DreERT2; IR, CD34-CreERT2; R26-tdTomato, Cdh5-dre; IR; Cd34-creER, Cd34-CreERT2; R26-tdTomato; Kdrflox, Cd34-CreERT2; R26-tdTomato; Pdgfraflox mice. Single-cell RNA sequence (scRNA-seq) was performed using the databases generated from our group and publicly data from NCBI Gene Expression (GEO) Omnibus (E6.5 to E18.5 cells). Additionally, whole-mount staining and three-dimensional reconstruction were used to depict different types of CD34+ progenitor distribution within transparent embryos.
Results: The proteomics unveiled an assumed intricate orchestration in developing human embryos from patients who experienced induced or spontaneous abortions. Our study demonstrated that the high level of CD34-expressing cells uncovered the potential naïve pluripotency to differentiate during embryonic development. Moreover, deletion of Kdr in CD34-derived cells resulted in stalled vessel
development in stages spanning E6.5 to E8.5, which is crucial to endothelium development. Also, the activation of the cell cycle machinery was identified as a driver of endothelial-to-hematopoietic transition (EHT) in Cd34+Runx1+Cd44+ cells from E9.5 to E11.5. Until late embryogenesis, CD34+ progenitors gave rise to fibroblasts. Finally, CD34+ cells contribute to the quiescent stem cell pool in the adult stage and enter the cell cycle when the body suffers damage.
Conclusions: Our results conducted a comprehensive study on the spatial and temporal induction of CD34+ cells and their intricate functions in embryonic development. These findings enlightened us on paving the way to innovatively tackle the complex hurdles present in stem cell and regenerative medicine, particularly in stem cell therapy.
Methods: Human embryos from PCW 6 to 12 were obtained from patients who had undergone induced or spontaneous abortions and samples were subjected to mass spectrometry. For the animal model, embryos were obtained among C57BL/6J, Cd34-DreERT2; IR, CD34-CreERT2; R26-tdTomato, Cdh5-dre; IR; Cd34-creER, Cd34-CreERT2; R26-tdTomato; Kdrflox, Cd34-CreERT2; R26-tdTomato; Pdgfraflox mice. Single-cell RNA sequence (scRNA-seq) was performed using the databases generated from our group and publicly data from NCBI Gene Expression (GEO) Omnibus (E6.5 to E18.5 cells). Additionally, whole-mount staining and three-dimensional reconstruction were used to depict different types of CD34+ progenitor distribution within transparent embryos.
Results: The proteomics unveiled an assumed intricate orchestration in developing human embryos from patients who experienced induced or spontaneous abortions. Our study demonstrated that the high level of CD34-expressing cells uncovered the potential naïve pluripotency to differentiate during embryonic development. Moreover, deletion of Kdr in CD34-derived cells resulted in stalled vessel
development in stages spanning E6.5 to E8.5, which is crucial to endothelium development. Also, the activation of the cell cycle machinery was identified as a driver of endothelial-to-hematopoietic transition (EHT) in Cd34+Runx1+Cd44+ cells from E9.5 to E11.5. Until late embryogenesis, CD34+ progenitors gave rise to fibroblasts. Finally, CD34+ cells contribute to the quiescent stem cell pool in the adult stage and enter the cell cycle when the body suffers damage.
Conclusions: Our results conducted a comprehensive study on the spatial and temporal induction of CD34+ cells and their intricate functions in embryonic development. These findings enlightened us on paving the way to innovatively tackle the complex hurdles present in stem cell and regenerative medicine, particularly in stem cell therapy.
More abstracts on this topic:
Bicaudal-C Homolog 1 Plays a Significant Role in Cardiac Development and Congenital Heart Defects
Liu Chia-feng, Leon Steven, Wessely Oliver, Tang Wai Hong
Birthweight is Associated with Left Ventricular Mass Index in ChildhoodSill Jordan, Woo Jessica, Urbina Elaine