Final ID: MP491
Clinical Effects of Acoramidis Versus Placebo in the ATTRibute-CM Study: Observations from the Intention-to-Treat Population
Abstract Body (Do not enter title and authors here): Introduction/Background: Acoramidis is a transthyretin (TTR) stabilizer providing near-complete (≥90%) TTR stabilization in vitro and is indicated for the treatment of wild type and variant TTR amyloid cardiomyopathy (ATTR-CM). In the Phase 3 ATTRibute-CM study (NCT03860935) in patients with ATTR-CM, acoramidis significantly improved the 4-step primary hierarchical outcome comprising mortality, morbidity, and physical function vs placebo. The original efficacy analysis considered all randomized patients but excluded those with impaired kidney function (modified intention-to-treat [mITT] population; N=611).
Research Questions/Hypothesis:
The efficacy of acoramidis in the overall study population (ITT population), including patients with kidney impairment (estimated glomerular filtration rate <30ml/min/1.73m2), has not been investigated and is assessed in the present analysis.
Methods/Approach: Relative reductions (RRs) in all-cause mortality (ACM), cardiovascular mortality, first all-cause hospitalization, first cardiovascular hospitalization, and first hospitalization for heart failure with acoramidis vs placebo were calculated. Least-squares mean (LSM) differences in 6-minute walk distance (6MWD) and Kansas City Cardiomyopathy Questionnaire–Overall Summary Score (KCCQ-OSS) were evaluated. RRs in achieving a ≥5% deterioration and ≥5% improvement in KCCQ-OSS with acoramidis vs placebo were assessed; a ≥5-point/≥5% change was considered as the threshold for a minimal clinically important difference for KCCQ-OSS.
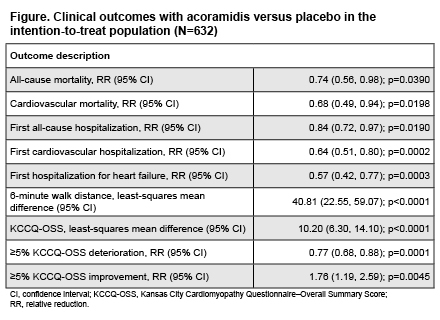
Results: In the ITT (n=421 acoramidis; n=211 placebo), acoramidis significantly improved all evaluated clinical outcomes vs placebo, with RRs (95% confidence interval [CI]) of 0.74 (0.56, 0.98) and 0.57 (0.42, 0.77) for ACM and first hospitalization for heart failure, respectively (Figure). 6MWD and KCCQ-OSS were improved with acoramidis compared with placebo, with LSM differences (95% CI) of 40.81 (22.55, 59.07) and 10.20 (6.30, 14.10), respectively. The RRs (95% CI) in achieving a ≥5% deterioration and ≥5% improvement in KCCQ-OSS with acoramidis vs placebo were 0.77 (0.68, 0.88) and 1.76 (1.19, 2.59).
Conclusion(s): The present ITT analysis showed consistent favorable results across all subgroups studied, confirming the efficacy of acoramidis in all patients with ATTR-CM, including patients with kidney impairment.
Research Questions/Hypothesis:
The efficacy of acoramidis in the overall study population (ITT population), including patients with kidney impairment (estimated glomerular filtration rate <30ml/min/1.73m2), has not been investigated and is assessed in the present analysis.
Methods/Approach: Relative reductions (RRs) in all-cause mortality (ACM), cardiovascular mortality, first all-cause hospitalization, first cardiovascular hospitalization, and first hospitalization for heart failure with acoramidis vs placebo were calculated. Least-squares mean (LSM) differences in 6-minute walk distance (6MWD) and Kansas City Cardiomyopathy Questionnaire–Overall Summary Score (KCCQ-OSS) were evaluated. RRs in achieving a ≥5% deterioration and ≥5% improvement in KCCQ-OSS with acoramidis vs placebo were assessed; a ≥5-point/≥5% change was considered as the threshold for a minimal clinically important difference for KCCQ-OSS.
Results: In the ITT (n=421 acoramidis; n=211 placebo), acoramidis significantly improved all evaluated clinical outcomes vs placebo, with RRs (95% confidence interval [CI]) of 0.74 (0.56, 0.98) and 0.57 (0.42, 0.77) for ACM and first hospitalization for heart failure, respectively (Figure). 6MWD and KCCQ-OSS were improved with acoramidis compared with placebo, with LSM differences (95% CI) of 40.81 (22.55, 59.07) and 10.20 (6.30, 14.10), respectively. The RRs (95% CI) in achieving a ≥5% deterioration and ≥5% improvement in KCCQ-OSS with acoramidis vs placebo were 0.77 (0.68, 0.88) and 1.76 (1.19, 2.59).
Conclusion(s): The present ITT analysis showed consistent favorable results across all subgroups studied, confirming the efficacy of acoramidis in all patients with ATTR-CM, including patients with kidney impairment.
More abstracts on this topic:
A Phase 2 Study Evaluating the Effects of Mivelsiran, an Investigational RNA Interference Therapeutic, on Hemorrhagic and Nonhemorrhagic Manifestations of Cerebral Amyloid Angiopathy
Greenberg Steven, Parikh Neal, Lee Jin-moo, Van Etten Ellis, Van Osch Matthias, Klijn Catharina, Sostelly Alexandre, Goteti Sasikiran, Sepehrband Farshid, Avbersek Andreja, Deering Robert
A Hypertrophic Cardiomyopathy-Based Model Estimate of the Prevalence of Danon Disease in the United StatesMaron Martin, Massera Daniele, Manganaro Susan, Bailey Miranda, Rehbein Fletcher, Taylor Matthew