Final ID: 4358910
Integrated Genomic Analysis Reveals Distinct Molecular Determinants of Human Fat Distribution
Abstract Body (Do not enter title and authors here): Background: Body fat distribution profoundly influences cardiometabolic risk, but the molecular drivers of regional fat accumulation remain incompletely defined. This study aimed to identify circulating analytes with genetically predicted effects on distinct fat depots and define biological pathways linked to each.
Research Question: Do circulating metabolites and proteins have genetically predicted effects on visceral (VAT), abdominal subcutaneous (ASAT), or gluteofemoral (GFAT) fat depots, and do these analytes reflect depot-specific biological mechanisms?
Methods: We evaluated UK Biobank participants with MRI-derived volumes of VAT, ASAT, and GFAT adjusted for BMI and height. Among 168 metabolites (N=22,630) and 2,910 proteins (N=5,023), we identified analytes significantly associated with one or more fat depots using linear regression adjusted for age, sex, batch, and time between imaging and blood draw. Protein pathway enrichment analysis was performed on significant proteins. Two-sample Mendelian randomization (MR) tested whether these analytes had genetically predicted effects on fat depot volumes; reverse MR assessed directionality. Associations between analytes and incident coronary artery disease (CAD) and type 2 diabetes (T2D) were evaluated using Cox models (N≈245K metabolomics; N≈46K proteomics).
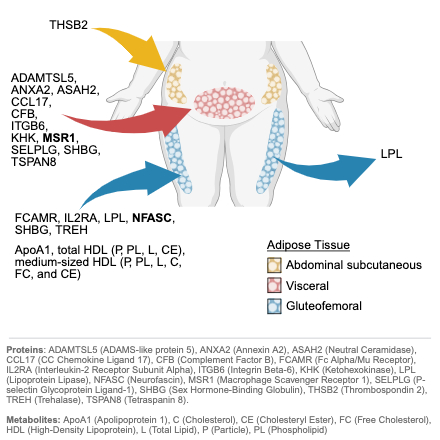
Results: Among participants with multi-omic data (mean age ~55 years; ~51% female), 839 analytes were associated with at least one fat depot (P<1e-4). MR identified 11 metabolites and 18 proteins with genetically predicted effects (P<0.05). Two proteins reached Bonferroni significance: macrophage scavenger receptor 1 (MSR1) with increased VAT (β=0.09, 95% CI: 0.05 to 0.12) and neurofascin (NFASC) with decreased GFAT (β=-0.08, 95% CI: -0.13 to -0.04). Reverse MR supported unidirectional effects for all but one analyte. Pathway enrichment revealed that VAT-associated proteins were enriched for inflammatory and immune signaling, GFAT for lipid metabolism and adipogenesis, and ASAT for complement and coagulation cascades. Multiple candidate analytes were associated with incident CAD and T2D over a median 11.2-year follow-up (P<1e-3).
Conclusion: Circulating analytes exhibit genetically anchored, depot-specific associations with fat distribution, enriched for inflammatory, metabolic, and immune-related pathways. These findings provide insight into fat depot biology and highlight candidate targets to therapeutically modulate regional adiposity and disease risk.
Research Question: Do circulating metabolites and proteins have genetically predicted effects on visceral (VAT), abdominal subcutaneous (ASAT), or gluteofemoral (GFAT) fat depots, and do these analytes reflect depot-specific biological mechanisms?
Methods: We evaluated UK Biobank participants with MRI-derived volumes of VAT, ASAT, and GFAT adjusted for BMI and height. Among 168 metabolites (N=22,630) and 2,910 proteins (N=5,023), we identified analytes significantly associated with one or more fat depots using linear regression adjusted for age, sex, batch, and time between imaging and blood draw. Protein pathway enrichment analysis was performed on significant proteins. Two-sample Mendelian randomization (MR) tested whether these analytes had genetically predicted effects on fat depot volumes; reverse MR assessed directionality. Associations between analytes and incident coronary artery disease (CAD) and type 2 diabetes (T2D) were evaluated using Cox models (N≈245K metabolomics; N≈46K proteomics).
Results: Among participants with multi-omic data (mean age ~55 years; ~51% female), 839 analytes were associated with at least one fat depot (P<1e-4). MR identified 11 metabolites and 18 proteins with genetically predicted effects (P<0.05). Two proteins reached Bonferroni significance: macrophage scavenger receptor 1 (MSR1) with increased VAT (β=0.09, 95% CI: 0.05 to 0.12) and neurofascin (NFASC) with decreased GFAT (β=-0.08, 95% CI: -0.13 to -0.04). Reverse MR supported unidirectional effects for all but one analyte. Pathway enrichment revealed that VAT-associated proteins were enriched for inflammatory and immune signaling, GFAT for lipid metabolism and adipogenesis, and ASAT for complement and coagulation cascades. Multiple candidate analytes were associated with incident CAD and T2D over a median 11.2-year follow-up (P<1e-3).
Conclusion: Circulating analytes exhibit genetically anchored, depot-specific associations with fat distribution, enriched for inflammatory, metabolic, and immune-related pathways. These findings provide insight into fat depot biology and highlight candidate targets to therapeutically modulate regional adiposity and disease risk.
More abstracts on this topic:
Accumulation of Epicardial Adipose Tissue as a Marker of Diastolic Dysfunction in Patients With Preserved Left Ventricular Ejection Fraction Undergoing Coronary Computed Tomography Angiograph
Ishikawa Hirotoshi, Kasayuki Noriaki, Fukuda Daiju, Otsuka Kenichiro, Sugiyama Takatoshi, Yamaura Hiroki, Hojo Kana, Kawa Yoshinori, Shintani Ako, Ito Asahiro, Yamazaki Takanori
A Novel Variant in GNB2 as a Cause of Sick Sinus SyndromeBulut Aybike, Karacan Mehmet, Saygili E. Alper, Pirli Dogukan, Aydin Eylul, Ozdemir Ozkan, Balci Nermin, Alanay Yasemin, Bilguvar Kaya, Akgun Dogan Ozlem