Final ID: Su1033
Integrative Metabolomic Analysis of Triglyceride-Independent Lipoprotein Lipase Pathway Mechanisms for Coronary Artery Disease
Abstract Body (Do not enter title and authors here): Background: Strong genetic evidence supports the associations between key genes in triglyceride (TG) metabolism via the lipoprotein lipase (LPL) pathway (i.e., LPL, APOC3, and ANGPTL3) and coronary artery disease (CAD). Given sparse human genetic or clinical trial evidence for other TG-modulating pathways, it is unclear if CAD risk reduction is due to reductions in TG levels alone or a secondary mechanism of action.
Aim: To determine if there are TG-independent mechanisms of CAD risk reduction specific to the LPL pathway using genomics and metabolomics.
Methods: We assessed the genetic effects of LPL pathway genes (67, 46, and 17 SNPs for LPL, APOC3, and ANGPTL3, respectively) and non-LPL pathway genes (3462 SNPs) for TG levels and 168 metabolites. We identified metabolites with associated effects that were greater than the associated effects with TG levels for LPL pathway genes and showed heterogeneity compared to the effects for non-LPL pathway genes. Cox proportional-hazards models were used to evaluate the association of candidate metabolites measured at baseline with incident CAD in participants of the UK Biobank. Models were adjusted for age, sex, TG levels, BMI, prevalent type 2 diabetes, statin, and ten PCs of genetic ancestry.
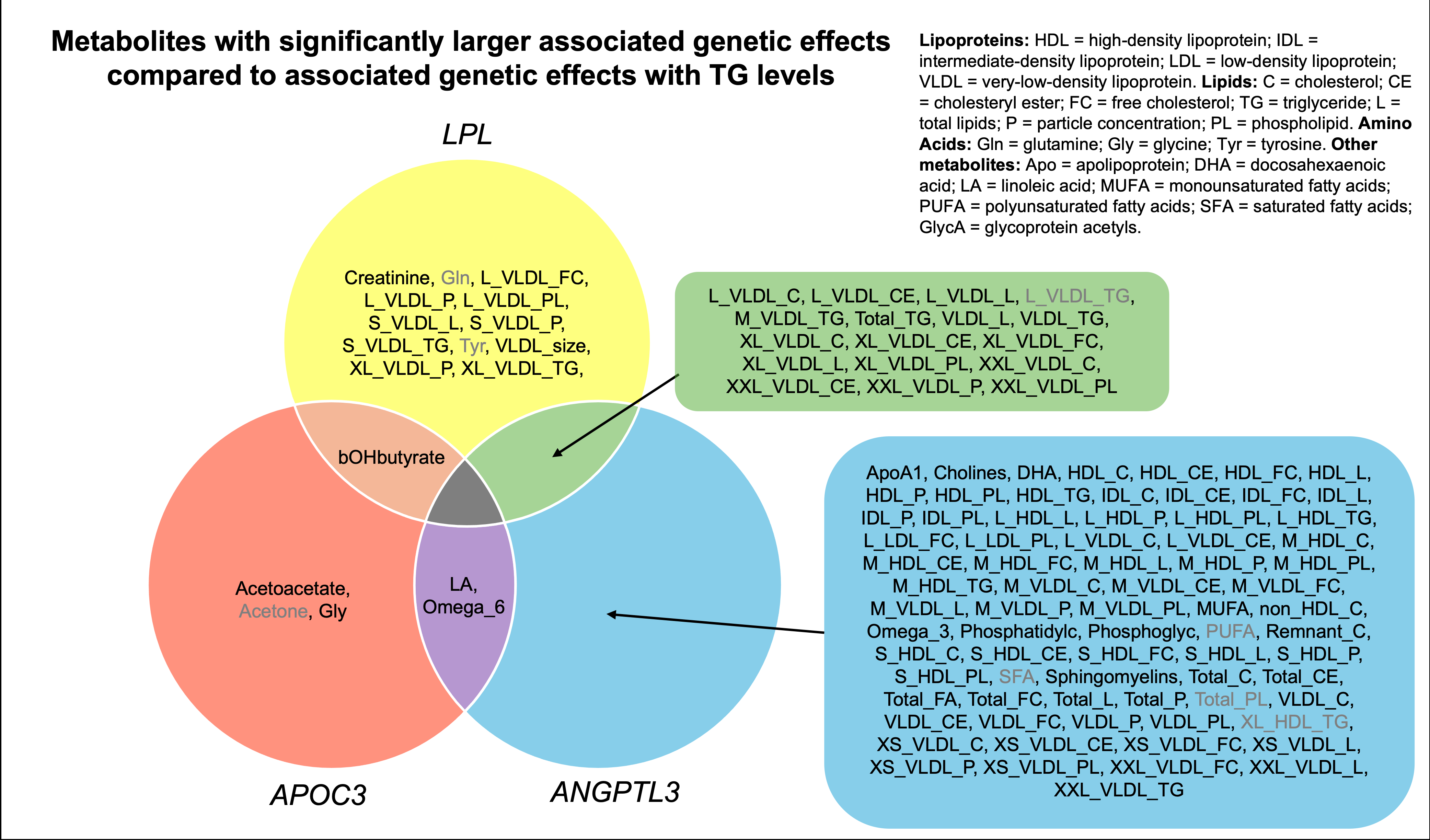
Results: Among 233,719 UK Biobank participants free of prevalent CAD and with metabolite measurements, the mean (SD) age was 57.1 (8.0) years, 105,111 (45.0%) were male, and all self-reported as white. Over a median (IQR) follow-up of 11.2 (10.5-11.9) years, there were 7992 (3.4%) incident CAD cases. There were 30, 6, and 93 metabolites with significantly greater genetic effects for LPL, APOC3, and ANGPTL3 than what was observed with TG levels, respectively. Among 20 metabolites with heterogeneity for ≥2 LPL pathway genes compared to non-LPL pathway genes, multi-variable Cox regression revealed 19 significantly associated with CAD (P<3x10-4).
Conclusion: LPL pathway genes have convergent and distinct impacts across metabolites. While LPL and ANGPTL3 have large genetic effects on VLDL metrics, APOC3 may have genetic effects beyond lipoprotein metabolism that impact CAD risk. The extent to which these metabolites influence CAD risk beyond TG alterations requires further study.
Aim: To determine if there are TG-independent mechanisms of CAD risk reduction specific to the LPL pathway using genomics and metabolomics.
Methods: We assessed the genetic effects of LPL pathway genes (67, 46, and 17 SNPs for LPL, APOC3, and ANGPTL3, respectively) and non-LPL pathway genes (3462 SNPs) for TG levels and 168 metabolites. We identified metabolites with associated effects that were greater than the associated effects with TG levels for LPL pathway genes and showed heterogeneity compared to the effects for non-LPL pathway genes. Cox proportional-hazards models were used to evaluate the association of candidate metabolites measured at baseline with incident CAD in participants of the UK Biobank. Models were adjusted for age, sex, TG levels, BMI, prevalent type 2 diabetes, statin, and ten PCs of genetic ancestry.
Results: Among 233,719 UK Biobank participants free of prevalent CAD and with metabolite measurements, the mean (SD) age was 57.1 (8.0) years, 105,111 (45.0%) were male, and all self-reported as white. Over a median (IQR) follow-up of 11.2 (10.5-11.9) years, there were 7992 (3.4%) incident CAD cases. There were 30, 6, and 93 metabolites with significantly greater genetic effects for LPL, APOC3, and ANGPTL3 than what was observed with TG levels, respectively. Among 20 metabolites with heterogeneity for ≥2 LPL pathway genes compared to non-LPL pathway genes, multi-variable Cox regression revealed 19 significantly associated with CAD (P<3x10-4).
Conclusion: LPL pathway genes have convergent and distinct impacts across metabolites. While LPL and ANGPTL3 have large genetic effects on VLDL metrics, APOC3 may have genetic effects beyond lipoprotein metabolism that impact CAD risk. The extent to which these metabolites influence CAD risk beyond TG alterations requires further study.
More abstracts on this topic:
A Blood(y) Pressure Crisis: Diffuse Alveolar Hemorrhage as a Rare Manifestation of Severely Uncontrolled Hypertension
Nandyal Shreyas, Amdetsion Gedion Yilma, Varma Revati, Kohli Saksham, Hammo Hasan
A Multicentre Study for Hands Only CPR (HOCPR) training assessment towards building a ‘Nation of Life Savers” in IndiaRavikumar Thanjavur, Sarma Kvs, Ravikumar Thanjavur, Sarkar Manuj, Debnath Dhrubajyoti, Behera Priyamadhaba, Ghate Jayshri, Trikha Divay, Samantaray A, Madhavi K