Final ID: MP1668
Predicting Adverse Cardiovascular Events in Patients Receiving Immune Checkpoint Inhibitors
Abstract Body (Do not enter title and authors here): Introduction: Immune checkpoint inhibitors (ICIs) have radically altered cancer therapy. Understanding adverse cardiovascular events (ACEs) associated with ICIs and identifying patients at risk remains crucial.
Methods: Data from a single center retrospective cohort was evaluated. The primary outcome was ACE, a composite of myocardial infarction (MI), coronary artery disease (CAD), stroke (CVA), peripheral vascular disease (PVD), myocarditis, heart failure, valvular disease, pericardial disease and arrhythmias. Secondary outcomes were individual components of ACE and all-cause mortality. Pre- and post-ICI imaging parameters from echocardiography (echo) and cardiac magnetic resonance (CMR) studies were evaluated. Cox regression analysis for ACE and all-cause mortality were also conducted on propensity-score matched populations.
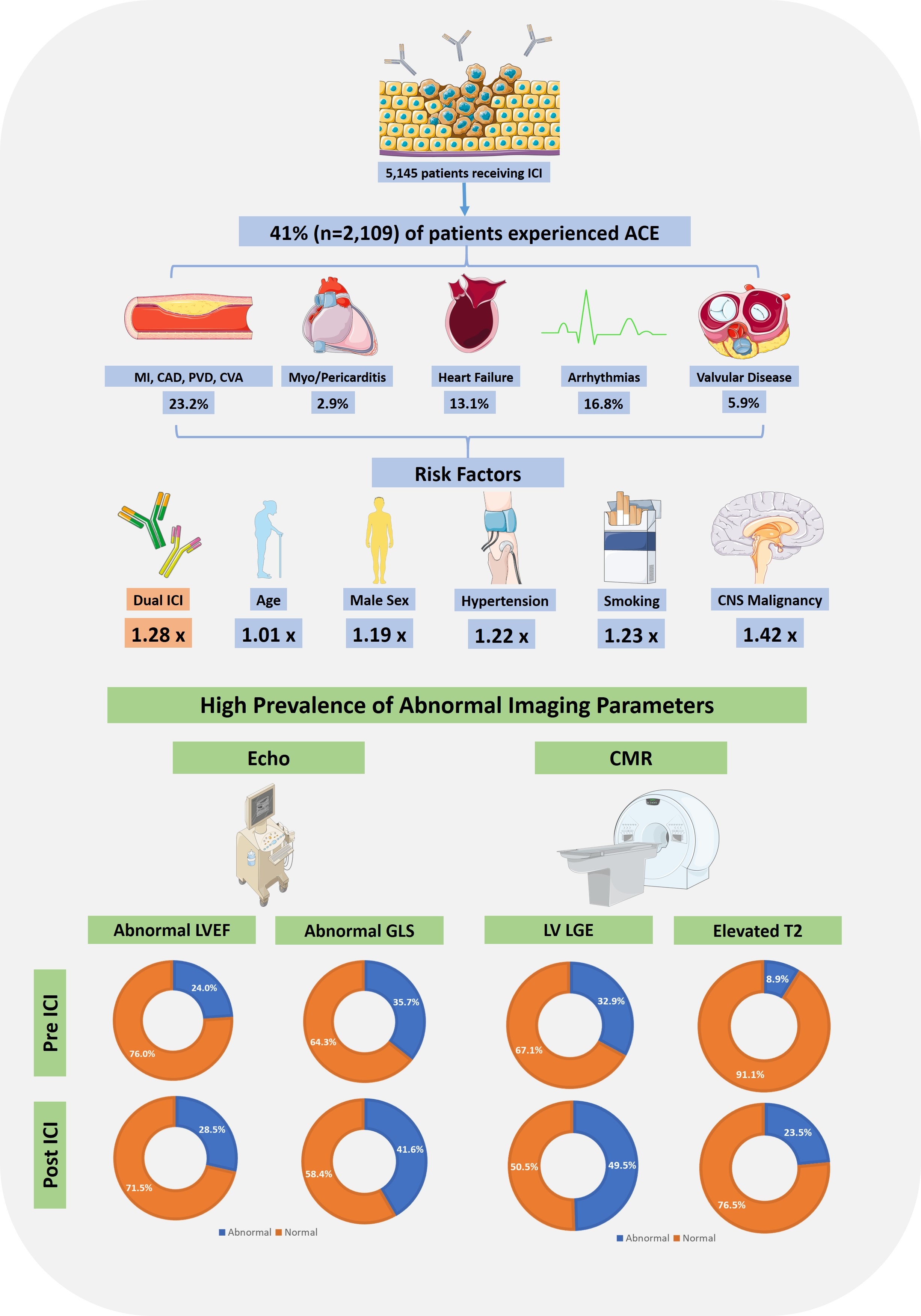
Results: 5,145 patients with cancer treated with ICIs, between 2013 and 2024, were included. 41% (n=2,109) of patients experienced ACE in median follow up time of 1.00 year (IQR 0.57-2.95 years), with MI/CAD/CVA/PVD (CVD) being the most common (n=1,194, 23.2%) (Figure 1). In multivariate analysis on propensity-score matched cohort (by age, gender, type of malignancy and pre-ICI ACE), dual ICI therapy (hazard ratio [HR] 1.28, confidence interval [CI] 1.12–1.46), age (HR 1.01, CI 1.00–1.02), male sex (HR 1.19, CI 1.01–1.42), smoking (HR 1.23, CI 1.02-1.47), hypertension (HR 1.22, CI 1.01-1.47), and central nervous system malignancy (HR 1.42, CI 1.08–1.87), were associated with ACE (Figure 2). Pre-ICI CVD was associated with lower risk of post-ICI ACE (HR 0.67, CI 0.55-0.81). CVD (HR 1.22, CI 1.02-1.46) and heart failure (HR 1.32, CI 1.08-1.61) were associated with increased risk of death (Figure 3). For patients with echo completed pre-ICI, 24.0% (n=338/1,405) had abnormal left ventricular ejection fraction (LVEF) and 35.7% (n=187/524) had abnormal global longitudinal strain (GLS), while post ICI 28.5% (n=464/1,626) had abnormal LVEF and 41.8% (n=272/652) abnormal GLS. Of patients with CMR pre-ICI, 32.9% (n=26/79) had left ventricular late gadolinium enhancement (LVLGE) and 8.9% (n=5/56) had abnormal T2 imaging; post ICI, 49.5% (n=110/222) had LVLGE and 23.5% (n=39/166) had abnormal T2 imaging.
Conclusion: ACE are common in patients receiving ICI; and pre-ICI CVD was associated increased risk of death. Further work, possibly guided by multimodality cardiac imaging, could identify patients at higher risk and guide preventative care.
Methods: Data from a single center retrospective cohort was evaluated. The primary outcome was ACE, a composite of myocardial infarction (MI), coronary artery disease (CAD), stroke (CVA), peripheral vascular disease (PVD), myocarditis, heart failure, valvular disease, pericardial disease and arrhythmias. Secondary outcomes were individual components of ACE and all-cause mortality. Pre- and post-ICI imaging parameters from echocardiography (echo) and cardiac magnetic resonance (CMR) studies were evaluated. Cox regression analysis for ACE and all-cause mortality were also conducted on propensity-score matched populations.
Results: 5,145 patients with cancer treated with ICIs, between 2013 and 2024, were included. 41% (n=2,109) of patients experienced ACE in median follow up time of 1.00 year (IQR 0.57-2.95 years), with MI/CAD/CVA/PVD (CVD) being the most common (n=1,194, 23.2%) (Figure 1). In multivariate analysis on propensity-score matched cohort (by age, gender, type of malignancy and pre-ICI ACE), dual ICI therapy (hazard ratio [HR] 1.28, confidence interval [CI] 1.12–1.46), age (HR 1.01, CI 1.00–1.02), male sex (HR 1.19, CI 1.01–1.42), smoking (HR 1.23, CI 1.02-1.47), hypertension (HR 1.22, CI 1.01-1.47), and central nervous system malignancy (HR 1.42, CI 1.08–1.87), were associated with ACE (Figure 2). Pre-ICI CVD was associated with lower risk of post-ICI ACE (HR 0.67, CI 0.55-0.81). CVD (HR 1.22, CI 1.02-1.46) and heart failure (HR 1.32, CI 1.08-1.61) were associated with increased risk of death (Figure 3). For patients with echo completed pre-ICI, 24.0% (n=338/1,405) had abnormal left ventricular ejection fraction (LVEF) and 35.7% (n=187/524) had abnormal global longitudinal strain (GLS), while post ICI 28.5% (n=464/1,626) had abnormal LVEF and 41.8% (n=272/652) abnormal GLS. Of patients with CMR pre-ICI, 32.9% (n=26/79) had left ventricular late gadolinium enhancement (LVLGE) and 8.9% (n=5/56) had abnormal T2 imaging; post ICI, 49.5% (n=110/222) had LVLGE and 23.5% (n=39/166) had abnormal T2 imaging.
Conclusion: ACE are common in patients receiving ICI; and pre-ICI CVD was associated increased risk of death. Further work, possibly guided by multimodality cardiac imaging, could identify patients at higher risk and guide preventative care.
More abstracts on this topic:
A Pressure-Volume Loops Approach Predicts Outcomes After Double Switch Operation For Congenitally Corrected Transposition Of The Great Arteries with Intact Ventricular Septum
Thatte Nikhil, Del Nido Pedro, Ghelani Sunil, Hammer Peter, Marx Gerald, Beroukhim Rebecca, Gauvreau Kimberlee, Callahan Ryan, Prakash Ashwin, Emani Sitaram, Hoganson David
A Competency-Based Screening Echocardiography Curriculum Designed for Rural American Indian Community Health RepresentativesThoroughman Rose, Riley Alan, De Loizaga Sarah, Adams David, Beaton Andrea, Buonfiglio Samantha, Danforth Kristen, Masyuko Sarah, Miller Mccall, Yadava Mrinal