Final ID: Su4010
Polygenic score analyses in a large multinational HCM clinical cohort identifies effects on disease penetrance and severity
Disease expressivity in hypertrophic cardiomyopathy (HCM) varies widely, ranging from unaffected genetically predisposed individuals to life-threatening complications. Polygenic scores (PGS) were shown to predict disease penetrance of HCM-causing rare genetic variants (HCMrv) and HCM-related outcomes in large biobanks. However, the utility of PGS in clinical cohorts remains unclear.
Research Questions
Can PGS predict disease penetrance in carriers of HCMrv in the clinical setting? Is PGS associated with disease severity and complications in individuals with HCM?
Methods
We studied a well-characterized clinical HCM cohort from Canada, Italy, the Netherlands and Spain, comprising 6,111 individuals affected by HCM and/or carrying a HCMrv. We used SBayesRC to derive a novel PGS for HCM from the largest published genome-wide association study. Standardized ancestry-adjusted PGS were calculated for all individuals and tested for association with HCM penetrance, maximal left ventricular wall thickness (MLVWT) and major adverse clinical events (MACE) using logistic, linear and Cox regression models, respectively, with adjustment for sex, rare variant status, site, and other covariates as relevant. MACE were defined as major ventricular arrhythmic or heart failure event, stroke, septal reduction therapy or all-cause mortality.
Results
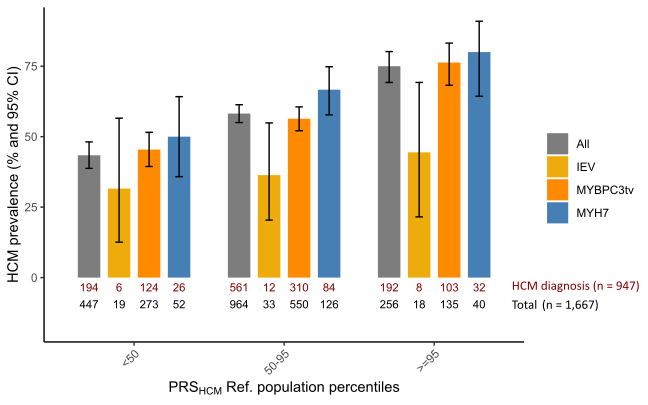
PGS was tested for association with HCM in the subset of 1,667 relatives carrying a HCMrv (age at last follow-up 47 ± 19, 49% female), of which 57% meet diagnostic criteria for HCM. PGS was associated with a diagnosis of HCM (Odds ratio 1.6 per standard deviation [SD] increase in PGS; 95% CI: 1.4-1.8). Male sex and hypertension also independently increased penetrance by 3-fold and 2-fold, respectively. HCM-penetrance increased with increasing PGS, in the entire set as well as in carriers of MYH7 pathogenic variants, MYBPC3 truncating variants, or intermediate effect variants (Figure). In 4,949 affected individuals (age at diagnosis 48 ± 17, 33% female, 49% carrying HCMrv), PGS was associated with disease severity. Each SD increase in PGS was associated with a 0.5 mm increase in MLVWT (95% CI: 0.3-0.6), and a 12% increase in lifetime risk of MACE (Hazard ratio 1.12, 95% CI: 1.06-1.18).
Conclusions
PGS assessment may enhance risk stratification and personalize monitoring strategies—guiding the timing, frequency, and scope of clinical evaluations in both genetically predisposed individuals and patients with manifest HCM.
- Jorda, Paloma ( Montreal Heart Institute, and Faculty of Medicine, Université de Montréal , Montreal , Quebec , Canada )
- Michels, Michelle ( Thorax Center, Erasmus University Medical Centre , Rotterdam , Netherlands )
- Christiaans, Imke ( University Medical Centre Groningen, University of Groningen , Groningen , Netherlands )
- Paterson, Natasha ( Tenaya Therapeutics , San Francisco , California , United States )
- Houweling, Arjan ( Amsterdam UMC , Amsterdam , Netherlands )
- Tremblay-gravel, Maxime ( Montreal Heart Institute, and Faculty of Medicine, Université de Montréal , Montreal , Quebec , Canada )
- Garceau, Patrick ( Montreal Heart Institute, and Faculty of Medicine, Université de Montréal , Montreal , Quebec , Canada )
- Lallier, Philippe ( Montreal Heart Institute, and Faculty of Medicine, Université de Montréal , Montreal , Quebec , Canada )
- Khan, Habib ( London Health Sciences Centre and Western University , London , Ontario , Canada )
- Roston, Thomas ( University of British Columbia , Vancouver , British Columbia , Canada )
- Steinberg, Christian ( Quebec Heart and Lung Institute and Université Laval , Quebec , Quebec , Canada )
- Lipov, Alex ( Amsterdam UMC, University of Amsterdam , Amsterdam , Netherlands )
- Olivotto, Iacopo ( Careggi University Hospital , Florence , Italy )
- Barriales, Roberto ( Complexo Hospitalario Universitario , A Coruña , Spain )
- Jurgens, Sean ( Amsterdam UMC, University of Amsterdam , Amsterdam , Netherlands )
- Cadrin-tourigny, Julia ( Montreal Heart Institute, and Faculty of Medicine, Université de Montréal , Montreal , Quebec , Canada )
- Tanck, Michael ( Amsterdam UMC, University of Amsterdam , Amsterdam , Netherlands )
- Amin, A.s. ( Amsterdam UMC, University of Amsterdam , Amsterdam , Netherlands )
- Adler, Arnon ( Peter Munk Cardiac Centre, University Health Network, University of Toronto , Toronto , Ontario , Canada )
- Tadros, Rafik ( Montreal Heart Institute, and Faculty of Medicine, Université de Montréal , Montreal , Quebec , Canada )
- Bezzina, Connie ( Amsterdam UMC, University of Amsterdam , Amsterdam , Netherlands )
- Gimeno Blanes, Juan Ramon ( University Hospital Virgen Arrixaca , Murcia , Spain )
- Castillo, Ismael ( Montreal Heart Institute, and Faculty of Medicine, Université de Montréal , Montreal , Quebec , Canada )
- Walsh, Roddy ( Cardiovascular & Genomics Research Institute, City St George's University of London , London , United Kingdom )
- Poel, Edwin ( Amsterdam UMC, University of Amsterdam , Amsterdam , Netherlands )
- Moussa, Samuel ( Montreal Heart Institute, and Faculty of Medicine, Université de Montréal , Montreal , Quebec , Canada )
- Haydarlou, Poeya ( Amsterdam UMC, University of Amsterdam , Amsterdam , Netherlands )
- Baas, Annette ( University Medical Centre Utrecht, Utrecht University , Utrecht , Netherlands )
Meeting Info:
Session Info:
Predicting and Treating Genetic Cardiomyopathies
Sunday, 11/09/2025 , 11:30AM - 12:30PM
Abstract Poster Board Session
More abstracts on this topic:
Nguyen Kevin, Cole John, Perry James, Gaynor Brady, Bai Zilong, Wang Fei, Xu Huichun, Leifer Dana
A Hypertrophic Cardiomyopathy Polygenic Score Modifies Penetrance of Pathogenic Hypertrophic and Dilated Cardiomyopathy Variants in Opposite DirectionsAbramowitz Sarah, Hoffman-andrews Lily, Depaolo John, Judy Renae, Owens Anjali, Damrauer Scott, Levin Michael
More abstracts from these authors:
Safabakhsh Sina, Raymond-paquin Alexandre, Jordà Paloma, Grondin Steffany, Parker Jeremy, Fazeli Amir, Castillo Ismael, Laksman Zachary, Tardif Jean-claude, Tadros Rafik
Prevalence and Risk Markers for Heart Failure in Hypertrophic Cardiomyopathy: A Multicenter Cross-Sectional Study With Central Assessment of Biomarkers and EchocardiogramsBigras Elizabeth, Roberts Jason, Tournoux Francois, Van Zyl Martin, Khan Habib, Oudit Gavin, Lee David, Roston Thomas, Steinberg Christian, Tremblay-gravel Maxime, Tadros Rafik, Boulet Jacinthe, Garceau Patrick, Lallier Philippe, Giraldeau Genevieve, Cadrin-tourigny Julia, Tardif Jean-claude, Fournier Anne, Joza Jacqueline