Final ID: MP2214
Comparing Cardiovascular Outcomes in New Users of Oral Semaglutide Versus Other Noninsulin Glucose-Lowering Therapies
Abstract Body (Do not enter title and authors here): Background Clinical trial and real-world evidence has shown the cardiovascular (CV) benefit from once-weekly semaglutide in people with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD). The recent SOUL clinical trial found that oral semaglutide was associated with significantly lower risk of major adverse cardiovascular events (MACE) compared with placebo in people with T2D and ASCVD. Less is known about the potential CV benefits of oral semaglutide in real-world settings.
Research question Among adults with T2D and ASCVD, is there a significant difference in the risk of MACE between those who initiated oral semaglutide vs those who initiated other second-line noninsulin glucose-lowering therapies (NIGLTs), specifically dipeptidyl peptidase 4 inhibitors (DPP-4is) or sodium-glucose cotransporter-2 inhibitors (SGLT2is)?
Goals/Aims We aimed to examine the incidence rate of 3-, 5-, and 2-point MACE, ischemic stroke (IS), and myocardial infarction (MI) among new users of oral semaglutide vs other NIGLTs.
Methods This observational cohort study used administrative claims from Optum Clinformatics Data Mart to compare the incidence of 3-, 5-, and 2-point MACE, IS, and MI among people with T2D and ASCVD who initiated oral semaglutide vs other NIGLTs. Further analyses compared CV outcomes in new users of oral semaglutide with those in matched new users of NIGLT classes: DPP-4is or SGLT2is. All comparison groups were balanced using propensity score matching.
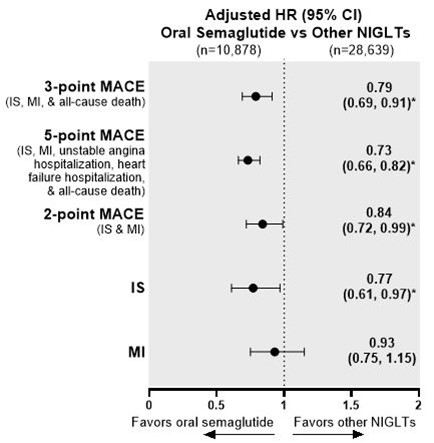
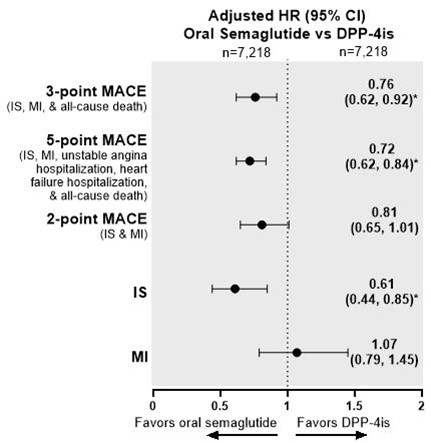
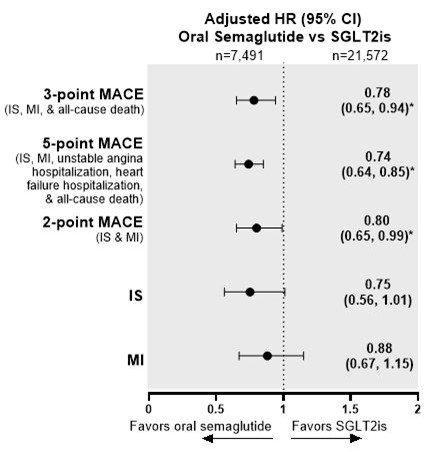
Results The oral semaglutide and other NIGLT matched cohorts comprised 10,878 and 28,639 people, respectively. People who initiated oral semaglutide had a significant risk reduction in 3-, 5-, and 2-point MACE and IS relative to people who initiated other NIGLTs (Figure 1). Relative to DPP-4i users, oral semaglutide users had significantly lower risk of 3- and 5-point MACE and IS (Figure 2). Compared with SGLT2i users, oral semaglutide users had significantly lower risk of 3-, 5-, and 2-point MACE (Figure 3).
Conclusions In a real-world setting, oral semaglutide was associated with reduced risk of CV outcomes compared with other NIGLTs, particularly DDP-4is and SGLT2is. These results complement clinical trial evidence of the CV benefit of oral semaglutide in people with T2D and ASCVD.
Research question Among adults with T2D and ASCVD, is there a significant difference in the risk of MACE between those who initiated oral semaglutide vs those who initiated other second-line noninsulin glucose-lowering therapies (NIGLTs), specifically dipeptidyl peptidase 4 inhibitors (DPP-4is) or sodium-glucose cotransporter-2 inhibitors (SGLT2is)?
Goals/Aims We aimed to examine the incidence rate of 3-, 5-, and 2-point MACE, ischemic stroke (IS), and myocardial infarction (MI) among new users of oral semaglutide vs other NIGLTs.
Methods This observational cohort study used administrative claims from Optum Clinformatics Data Mart to compare the incidence of 3-, 5-, and 2-point MACE, IS, and MI among people with T2D and ASCVD who initiated oral semaglutide vs other NIGLTs. Further analyses compared CV outcomes in new users of oral semaglutide with those in matched new users of NIGLT classes: DPP-4is or SGLT2is. All comparison groups were balanced using propensity score matching.
Results The oral semaglutide and other NIGLT matched cohorts comprised 10,878 and 28,639 people, respectively. People who initiated oral semaglutide had a significant risk reduction in 3-, 5-, and 2-point MACE and IS relative to people who initiated other NIGLTs (Figure 1). Relative to DPP-4i users, oral semaglutide users had significantly lower risk of 3- and 5-point MACE and IS (Figure 2). Compared with SGLT2i users, oral semaglutide users had significantly lower risk of 3-, 5-, and 2-point MACE (Figure 3).
Conclusions In a real-world setting, oral semaglutide was associated with reduced risk of CV outcomes compared with other NIGLTs, particularly DDP-4is and SGLT2is. These results complement clinical trial evidence of the CV benefit of oral semaglutide in people with T2D and ASCVD.
More abstracts on this topic:
A Novel Animal Model for Pulmonary Hypertension: Lung Endothelial Specific Deletion of Egln1 in Mice
Liu Bin, Yi Dan, Ramirez Karina, Fallon Michael, Dai Zhiyu
A Multicenter, Prospective, Randomized Controlled Trial of Endovascular Treatment with or without Intravenous ThromBolysis in Acute Ischemic Stroke of Basilar Artery Occlusion (BEST-BAO): Study ProtocolXiang Yang, Siddiqui Adnan, Yang Shu, Mocco J, Yu Nengwei, Schonewille Wouter, Guo Fuqiang