Final ID: Mo3132
A 3-Year, Pre-Trial, Real-world Data Analysis of Patients Enrolled in VICTORION-INITIATE: Insights Using Tokenization
Abstract Body (Do not enter title and authors here): Background: VICTORION-INITIATE (NCT04929249) showed significantly greater LDL-C lowering in patients (pts) with ASCVD with an inclisiran first (IF) strategy (adding inclisiran immediately on failure to achieve LDL-C <70 mg/dL on maximally tolerated statin therapy) vs usual care (UC). Tokenization can be leveraged to link clinical trial data to pt-level real-world data (RWD) to provide a comprehensive overview of the pt journey pre- and post-trial and enhance understanding of clinical trial and long-term pt outcomes.
Aim: To utilize tokenization and linked RWD to characterize the pt journey pre-VICTORION-INITIATE enrollment.
Methods: Eligible pts from VICTORION-INITIATE were deidentified and tokenized with unique IDs, allowing linking to RWD (EMR, claims, hospital, and lab records) to follow the pt journey in the 3-year pre-trial and 2-year post-trial periods. Here we present demographics, clinical characteristics, and lipid-lowering therapy (LLT) use of pts with linked pre-trial RWD.
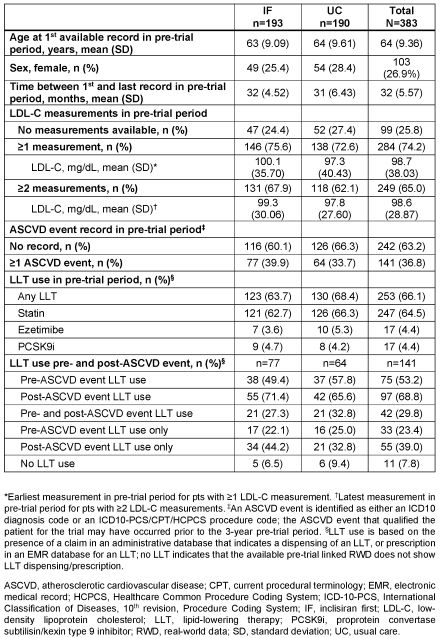
Results: Of 450 pts randomized, 383 (85.1%) were linked with pre-trial RWD (IF arm, n=193; UC arm, n=190); 26.9% were female, first available recorded mean (SD) age was 64 (9.36) years. Among the 74.2% (n=284) of pts with linked, pre-trial, lipid test RWD, mean (SD) earliest and latest LDL-C values were 100.1 mg/dL (35.70) and 99.3 mg/dL (30.06), respectively, for pts in the IF arm vs 97.3 mg/dL (40.43) and 97.8 mg/dL (27.60) for pts in the UC arm (Table). Overall, in the pre-trial period, LLT was dispensed/prescribed to 66.1% of pts, statins to 64.5%, ezetimibe to 4.4%, and PCSK9i to 4.4%. Of pts with ≥1 recorded ASCVD events in the pre-trial period (n=141), 53.2% and 68.8% had available LLT dispensing/prescription records pre- and post-event, respectively.
Conclusions: In the 3 years pre-VICTORION-INITIATE, mean LDL-C levels were above recommended goals, non-statin LLT prescription was infrequent, and ~31% of pts with ≥1 recorded ASCVD event had no evidence of post-event LLT use, highlighting a need for more aggressive LDL-C management. Full data visibility for each pt is a limitation of this approach. Tokenization post-trial will allow for long-term evaluation of LLT practices.
Aim: To utilize tokenization and linked RWD to characterize the pt journey pre-VICTORION-INITIATE enrollment.
Methods: Eligible pts from VICTORION-INITIATE were deidentified and tokenized with unique IDs, allowing linking to RWD (EMR, claims, hospital, and lab records) to follow the pt journey in the 3-year pre-trial and 2-year post-trial periods. Here we present demographics, clinical characteristics, and lipid-lowering therapy (LLT) use of pts with linked pre-trial RWD.
Results: Of 450 pts randomized, 383 (85.1%) were linked with pre-trial RWD (IF arm, n=193; UC arm, n=190); 26.9% were female, first available recorded mean (SD) age was 64 (9.36) years. Among the 74.2% (n=284) of pts with linked, pre-trial, lipid test RWD, mean (SD) earliest and latest LDL-C values were 100.1 mg/dL (35.70) and 99.3 mg/dL (30.06), respectively, for pts in the IF arm vs 97.3 mg/dL (40.43) and 97.8 mg/dL (27.60) for pts in the UC arm (Table). Overall, in the pre-trial period, LLT was dispensed/prescribed to 66.1% of pts, statins to 64.5%, ezetimibe to 4.4%, and PCSK9i to 4.4%. Of pts with ≥1 recorded ASCVD events in the pre-trial period (n=141), 53.2% and 68.8% had available LLT dispensing/prescription records pre- and post-event, respectively.
Conclusions: In the 3 years pre-VICTORION-INITIATE, mean LDL-C levels were above recommended goals, non-statin LLT prescription was infrequent, and ~31% of pts with ≥1 recorded ASCVD event had no evidence of post-event LLT use, highlighting a need for more aggressive LDL-C management. Full data visibility for each pt is a limitation of this approach. Tokenization post-trial will allow for long-term evaluation of LLT practices.
More abstracts on this topic:
Assessing Cardiovascular Risk in Patients with New Onset Diabetes After Statin Initiation
Schaaf Lucas, Nadar Priyanka, Gebrehiwot Ledya, Jean-marie Elizabeth, Simon Steven, Rosenberg Michael
AI Integration Decreased Rural Documentation Burden by 40% in Medicare's Chronic Care Management SettingMiller Jered, Jimmerson Garrett, Miller Callie, Dey Ashley, Wheeler Caroline, Miller Samuel, Al Tibi Ghaith, Chronos Nicolas