Final ID: MP441
Effect of Caloric Restriction on Cardiovascular-Kidney-Metabolic Health in Young Adults: Insights from the CALERIE Trial
Abstract Body (Do not enter title and authors here): Background: Cardiovascular-kidney-metabolic (CKM) health impairments are common among young adults in the US and contribute to premature morbidity and mortality, but the extent to which their onset, progression, and regression is modifiable through dietary interventions is uncertain.
Research Question: How does caloric restriction (CR) impact CKM health among young adults?
Methods: CALERIE (Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy) was a phase 2 multicenter, randomized trial evaluating the effects of CR compared with a control (ad libitum) diet among healthy young adults with body mass index (BMI) 22-27.9 kg/m2. CKM health was defined using the American Heart Association CKM syndrome framework, and treatment effects of CR on CKM syndrome stage transitions and individual CKM risk factors were evaluated.
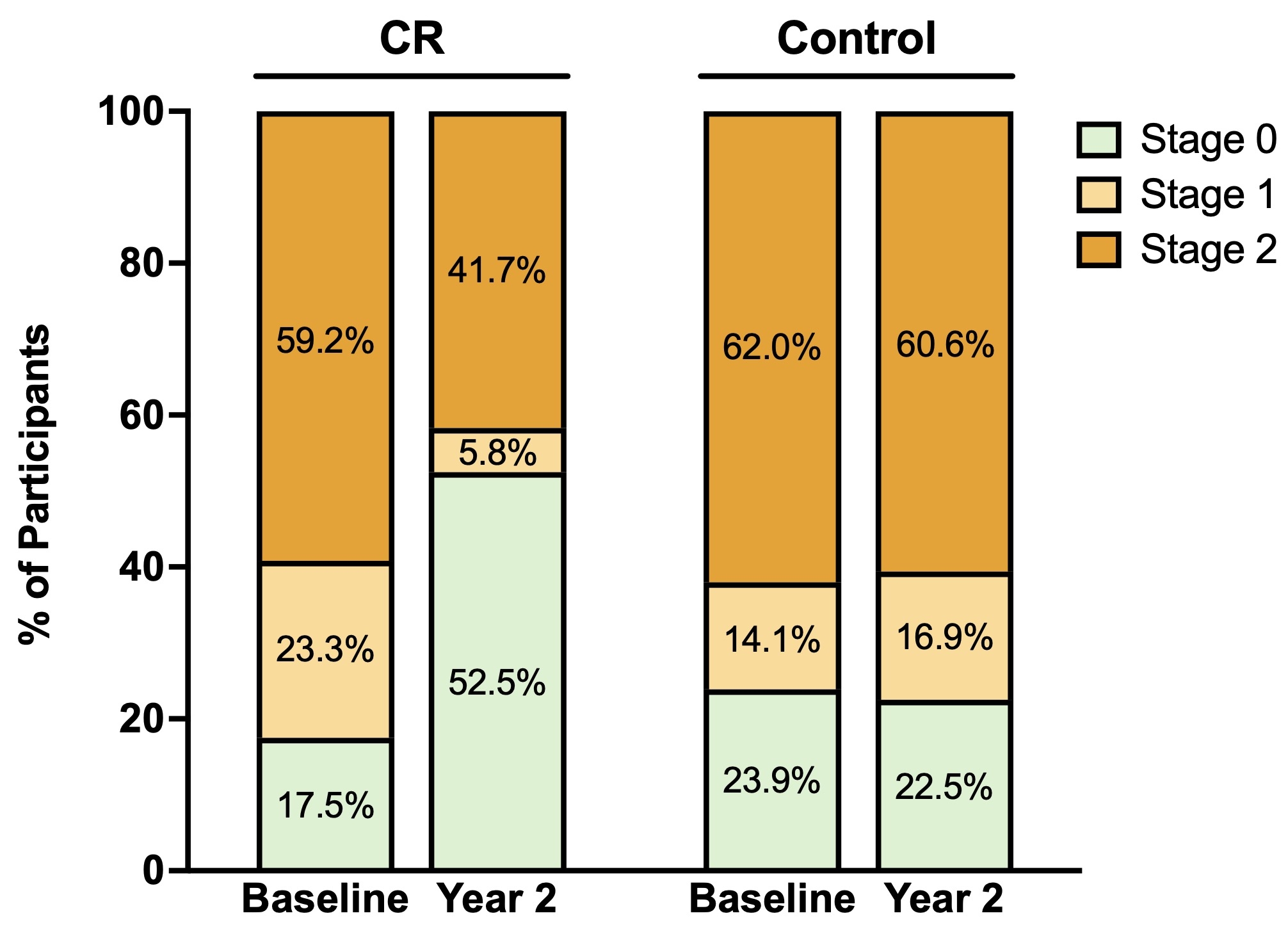
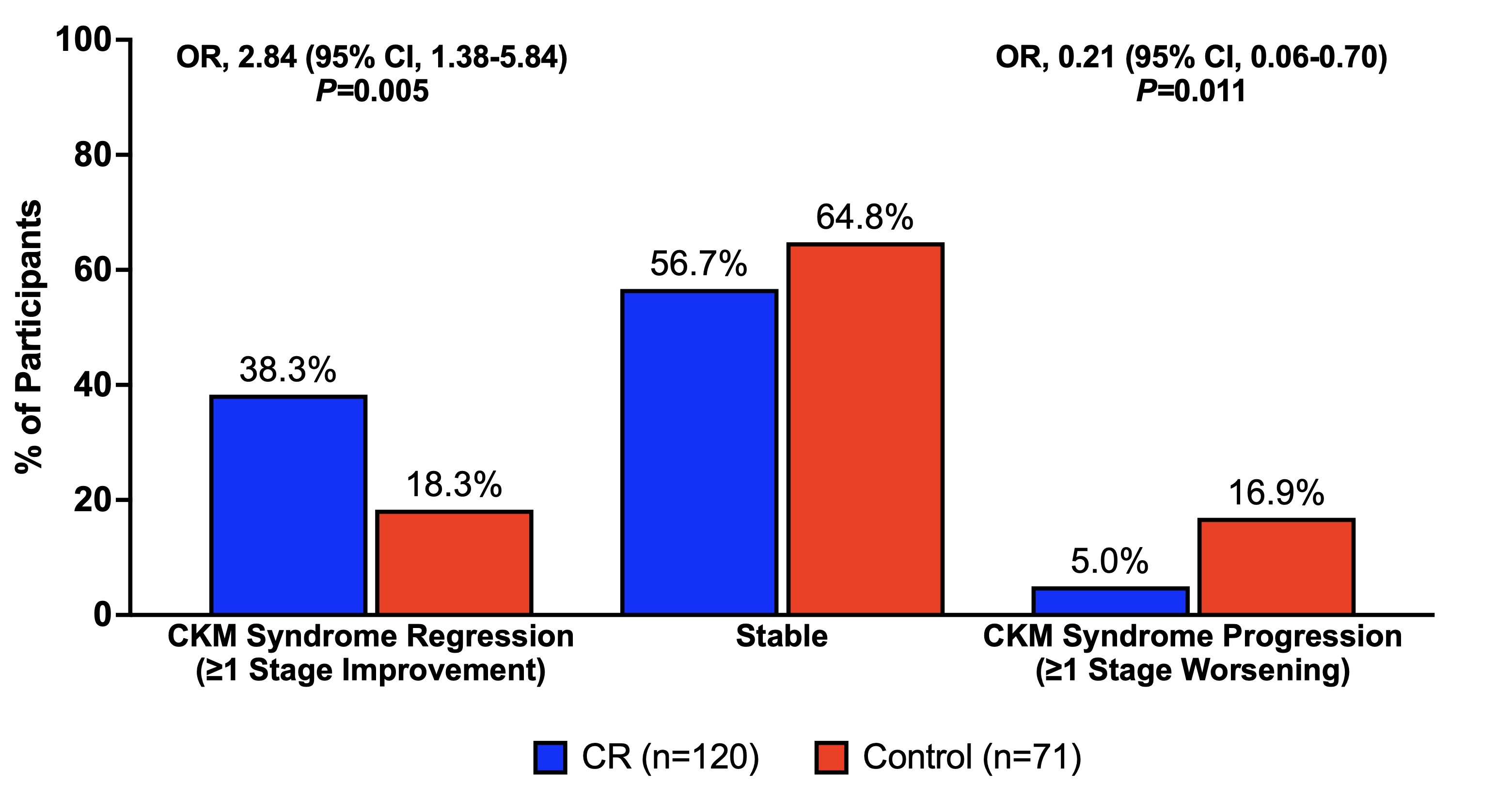
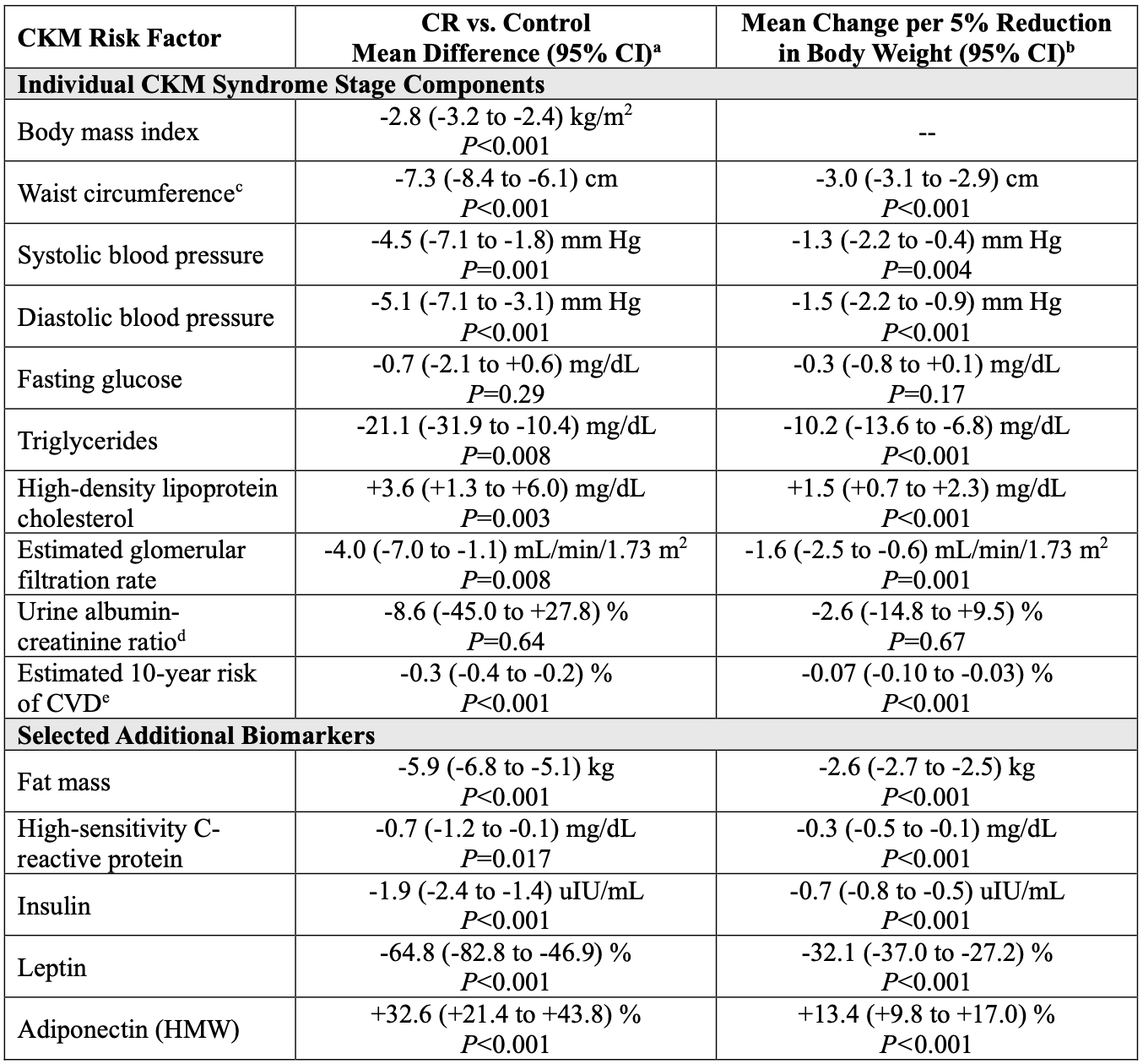
Results: Of 220 participants randomized 2:1 to CR or control, 191 (87%) completed the trial and were included in the analysis (mean age, 38±7 years; 70% female; mean BMI, 25.2±1.7 kg/m2). Those in the CR group achieved a mean CR of 11.9% (95% CI, 11.0, 12.8%), compared with 0.8% (95% CI, -0.7, 2.4%) in the control group. At baseline, 38 (20%), 38 (20%), and 115 (60%) had CKM syndrome stages 0, 1, and 2, respectively. After 2 years, CR was associated with lower odds of CKM syndrome progression compared with control (5% vs. 17%; odds ratio [OR], 0.21; 95% CI, 0.06, 0.70; P=0.011). Among those with CKM syndrome stage 0 at baseline, CR additionally reduced incident CKM syndrome (14% vs. 65%; OR, 0.09; 95% CI, 0.02, 0.44; P=0.003) by 2 years. CR additionally appeared to promote CKM syndrome regression (38% vs. 18%; OR, 2.84; 95% CI, 1.38, 5.84; P=0.005) and remission (38% vs. 14%; OR, 3.74; 95% CI, 1.72, 8.14; P=0.001) (Figure). CR improved several CKM risk factors, including body weight (mean change, -11.1%; 95% CI, -12.6, -9.5%; P<0.001), body mass index, waist circumference, blood pressure, triglyceride levels, and high-density lipoprotein cholesterol; improvements in several CKM risk factors were associated with the magnitude of body weight change between baseline and 2 years (Table).
Conclusions: Among putatively healthy young adults without BMI-defined obesity in the CALERIE trial, 80% had evidence of CKM health impairments. Moderate CR reduced progression and enhanced regression of CKM syndrome, underscoring its potential to promote optimal CKM health during early life stages.
Research Question: How does caloric restriction (CR) impact CKM health among young adults?
Methods: CALERIE (Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy) was a phase 2 multicenter, randomized trial evaluating the effects of CR compared with a control (ad libitum) diet among healthy young adults with body mass index (BMI) 22-27.9 kg/m2. CKM health was defined using the American Heart Association CKM syndrome framework, and treatment effects of CR on CKM syndrome stage transitions and individual CKM risk factors were evaluated.
Results: Of 220 participants randomized 2:1 to CR or control, 191 (87%) completed the trial and were included in the analysis (mean age, 38±7 years; 70% female; mean BMI, 25.2±1.7 kg/m2). Those in the CR group achieved a mean CR of 11.9% (95% CI, 11.0, 12.8%), compared with 0.8% (95% CI, -0.7, 2.4%) in the control group. At baseline, 38 (20%), 38 (20%), and 115 (60%) had CKM syndrome stages 0, 1, and 2, respectively. After 2 years, CR was associated with lower odds of CKM syndrome progression compared with control (5% vs. 17%; odds ratio [OR], 0.21; 95% CI, 0.06, 0.70; P=0.011). Among those with CKM syndrome stage 0 at baseline, CR additionally reduced incident CKM syndrome (14% vs. 65%; OR, 0.09; 95% CI, 0.02, 0.44; P=0.003) by 2 years. CR additionally appeared to promote CKM syndrome regression (38% vs. 18%; OR, 2.84; 95% CI, 1.38, 5.84; P=0.005) and remission (38% vs. 14%; OR, 3.74; 95% CI, 1.72, 8.14; P=0.001) (Figure). CR improved several CKM risk factors, including body weight (mean change, -11.1%; 95% CI, -12.6, -9.5%; P<0.001), body mass index, waist circumference, blood pressure, triglyceride levels, and high-density lipoprotein cholesterol; improvements in several CKM risk factors were associated with the magnitude of body weight change between baseline and 2 years (Table).
Conclusions: Among putatively healthy young adults without BMI-defined obesity in the CALERIE trial, 80% had evidence of CKM health impairments. Moderate CR reduced progression and enhanced regression of CKM syndrome, underscoring its potential to promote optimal CKM health during early life stages.
More abstracts on this topic:
Albuminuria Drives Type 2 Diabetes-Related Atrial Fibrillation: an ACCORD substudy
Siqueira Amanda, Everett Brendan
Acculturation and Cardiovascular-Kidney-Metabolic Syndrome: a Study of Immigrant Adults From the National Health and Nutrition Examination SurveyChakrabarti Amit, Le Austin, Elfassy Tali, Yang Eugene