Final ID: 4342855
Comparative Effectiveness of Cardiac Rehabilitation After Surgical Aortic Valve Replacement – A Target Trial Emulation
Cardiac rehabilitation (CR) after surgical aortic valve replacement (SAVR) is recommended by guidelines, but participation is low. This is partly because the supporting evidence is weak: prior trials were small with short follow-up and few clinical events. Because CR is guideline-recommended, randomized trials of CR vs. no CR would not be ethical, and high-quality observational comparative effectiveness studies are urgently needed.
Research Question
Does CR reduce the risk of death and major adverse cardiovascular events (MACE, defined as acute myocardial infarction, stroke, or heart failure) in Medicare beneficiaries undergoing SAVR?
Methods
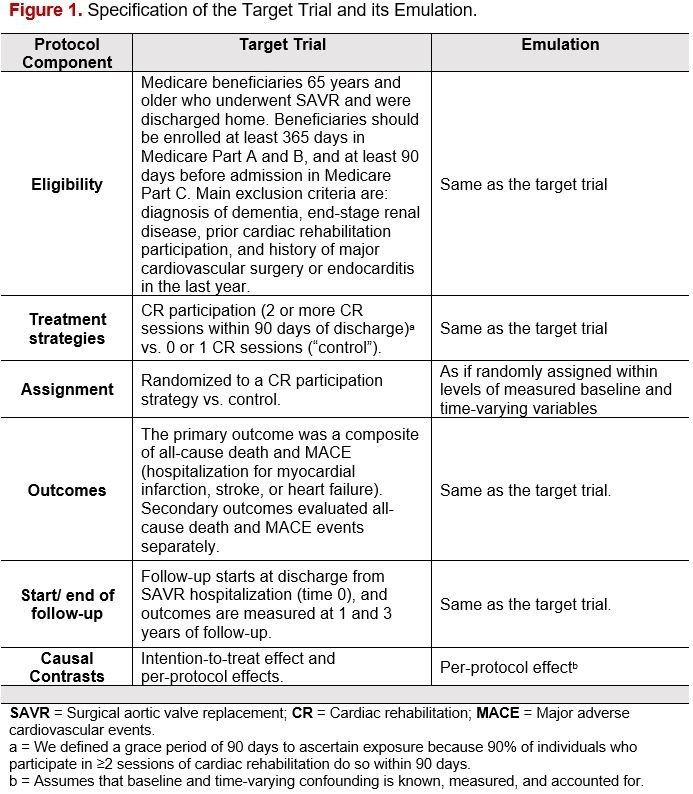
We first specified a target trial – a hypothetical pragmatic randomized trial that would answer the causal question of interest – and then emulated the target trial using 100% Medicare claims (Fig. 1). We included Medicare beneficiaries aged ≥65 years who underwent SAVR between 10/2016 and 12/2022. The intervention (“CR participation”) was defined as receipt of ≥2 CR sessions within 90 days of discharge, compared with receipt of 0 or 1 CR session (“control”). The cloning-censoring-weighting approach was used to align the time for determining eligibility, treatment assignment, and start of follow-up, and accounted for baseline and time-varying covariates. The primary outcome was death from any cause or hospitalization for MACE. Two falsification endpoints (new cancer; acute infection) were used to test for residual confounding.
Results
Among 44,136 Medicare beneficiaries, 48.5% participated in ≥2 CR sessions after SAVR (Fig. 2). CR participants were more likely to be male and identify as Non-Hispanic White, and less likely to be dually enrolled in Medicare and Medicaid or reside in neighborhoods with the highest social vulnerability.
CR participants had a lower risk of the primary outcome at 3 years compared with the control (17.5% vs 20.1%; adjusted risk difference, -2.6 pp; 95% CI, -3.5 pp to -1.7 pp). Analyses of falsification endpoints were compatible with the absence of strong residual confounding (Fig. 3).
Discussion
In the largest and most diverse study of CR after SAVR, patients with CR participation had a lower risk of death or MACE at 3 years. Fewer than half of Medicare beneficiaries participated in CR after SAVR, with marked socioeconomic inequities. Our findings highlight the urgent need for strategies to equitably improve participation in CR after SAVR.
- Rr Decker, Sergio ( Smith Center for Outcomes Research, Beth Israel Deaconess Medical Center and Harvard Medical School. , Boston , Massachusetts , United States )
- Rosa, Regis ( Internal Medicine Department, Moinhos de Vento Hospital and Faculdade de Medicina, Universidade Federal do Rio Grande do Sul , Porto Alegre , Brazil )
- Yeh, Robert ( Smith Center for Outcomes Research, Beth Israel Deaconess Medical Center and Harvard Medical School , Boston , Massachusetts , United States )
- Sperling, Laurence ( Division of Cardiology, Department of Medicine, Emory Clinical Cardiovascular Research Institute; and Emory University School of Medicine , Atlanta , Georgia , United States )
- Fonarow, Gregg ( Division of Cardiology, Ahmanson-UCLA Cardiomyopathy Center, University of California Los Angeles Medical Center , Los Angeles , California , United States )
- Keteyian, Steven ( Division of Cardiovascular Medicine, Henry Ford Medical Group , Detroit , Michigan , United States )
- Beatty, Alexis ( Department of Epidemiology and Biostatistics and Department of Medicine, Division of Cardiology, University of California, San Francisco , San Francisco , California , United States )
- Dahabreh, Issa ( CAUSALab, Department of Epidemiology and Department of Biostatistics, Harvard T.H. Chan School of Public Health , Boston , Massachusetts , United States )
- Kazi, Dhruv ( Smith Center for Outcomes Research, Beth Israel Deaconess Medical Center and Harvard Medical School , Boston , Massachusetts , United States )
- Essa, Mohammed ( Smith Center for Outcomes Research, Beth Israel Deaconess Medical Center and Harvard Medical School , Boston , Massachusetts , United States )
- Song, Yang ( Smith Center for Outcomes Research, Beth Israel Deaconess Medical Center , Boston , Massachusetts , United States )
- Liang, Lichen ( Smith Center for Outcomes Research, Beth Israel Deaconess Medical Center , Boston , Massachusetts , United States )
- Inoue, Kosuke ( Department of Social Epidemiology, Graduate School of Medicine, Kyoto University, Kyoto, Japan; Hakubi Center, Kyoto University , Kyoto , Japan )
- Mcconeghy, Kevin ( Department of Health Services, Policy, and Practice, Brown University School of Public Health, Providence, RI; Center of Innovation Long-Term Services and Supports, Providence Veterans Administration Medical Center , Providence , Rhode Island , United States )
- Wu, Wen-chih ( Providence VA Medical Center and Cardiovascular Institute, Brown University Health. Departments of Medicine and Epidemiology, Brown University , Providence , Rhode Island , United States )
- Varghese, Merilyn ( Department of Internal Medicine, Section of Cardiovascular Medicine, Yale School of Medicine, New Haven, CT, USA; Department of Cardiology, Veterans Affairs Connecticut Healthcare System , West Haven , Connecticut , United States )
- Thompson, Mike ( Department of Cardiac Surgery, Michigan Medicine, Ann Arbor, MI; Center for Healthcare Outcomes and Policy, University of Michigan , Ann Arbor , Michigan , United States )
Meeting Info:
Session Info:
QCOR Early Career Investigator Abstract Award Competition
Friday, 11/07/2025 , 11:00AM - 12:15PM
Abstract Oral Session
More abstracts on this topic:
Zheng Jimmy, Rodriguez Fatima, Eng David, Sandhu Alexander, Alkan Eren, Deshpande Aniruddha, Efobi Jo Ann, Fearon William, Heidenreich Paul, Khandwala Nishith, Maron David, Fernandes Cordeiro De Morais Felipe
Body Mass Index and Waist-Hip Ratio -- Risk Factors for Aortic Valve Disease: The Atherosclerosis Risk in Communities (ARIC) StudyZhang Chunxiao, Moser Ethan, Eaton Anne, Van't Hof Jeremy, Tang Weihong, Shah Amil, Folsom Aaron, Chen Lin
More abstracts from these authors:
Kochar Ajar, Liang Lichen, Almarzooq Zaid, Song Yang, Kazi Dhruv, Secemsky Eric, Yeh Robert
Long-term Cardiovascular Outcomes After COVID-19 Hospitalization in the Omicron Era: Retrospective Cohort StudyDa Rosa Decker Sergio Renato, Song Yang, Dahabreh Issa, Yeh Robert, Kazi Dhruv