Final ID: MP164
Hyperkalemia Sequelae in Patients With Chronic Kidney Disease, Heart Failure, Neither or Both: Findings From the TRACK Study
Methods: TRACK enrolled patients with serum K+ >5.0 mmol/L in Germany, Italy, Spain, the UK, and the US. Data were gathered from participants’ medical records at 3-month intervals on therapeutic objectives, treatment regimens, K+ normalization rates, continuation of RAASi and mineralocorticoid receptor antagonist (MRA) therapy, and clinical outcomes. All participants provided informed consent. We conducted descriptive statistical analyses to identify trends between participants with CKD, HF, neither, or both.
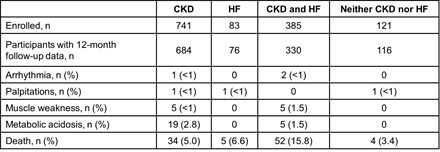
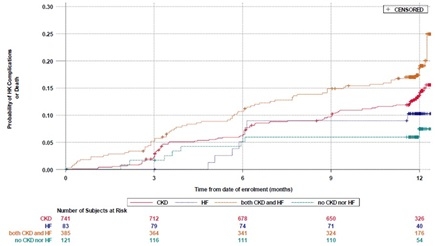
Results: Of 1330 TRACK participants, 741 had CKD at baseline, 83 HF, 385 both, and 121 neither. Mean age was 68±14 years, 31% were female, 8% Latino, 66% White, 29% Black, and 1% Asian. At baseline, ACE/ARB/ARNI and MRA use, respectively, was 51% and 3% among patients with CKD; 84% and 51% for those with HF; 62% and 28% for those with both; 60% and 6% for those with neither (P=0.0006 for ACE/ARB/ARNI use among the four groups and P<0.0001 for MRA). Dose adjustment was infrequent. K+ binder initiation or dose increase was reported for 12%, 3%, 16%, and 0% of those with CKD, HF, both, or neither, respectively (P=0.0008 for K+ binder initiation/dose increase among the four groups). Metabolic acidosis and death were the most common sequelae of HK (Table); causes of death included renal, cardiac, and multisystem failure, infection, and cancer. Occurrence of any HK complication or death was similar in patients with CKD (event rate at 12 months: 13.6 [95% CI 11.1, 16.2], P=0.09) or HF alone (10.3 [95% CI 3.5, 17.0], P=0.08) versus those with CKD and HF (18.6 [95% CI 14.6, 22.6]). Complications/death were more frequent among patients with CKD and HF versus those with neither (7.5 [95% CI 2.3, 12.6], P=0.0123) (Figure).
Conclusion: Patients with HK with CKD and HF are at particularly high risk for poor outcomes. More consistent guideline-directed HK management including K+ binder use is needed to improve current suboptimal use of potentially life-saving CKD and HF therapies.
- Hsia, Judith ( Univ of Colorado , Aurora , Colorado , United States )
- Ferraro, Pietro Manuel ( Università degli Studi di Verona , Verona , Italy )
- Butler, Javed ( Baylor Scott and White Research , Dallas , Texas , United States )
- Bishop, Meredith ( ASTRAZENECA , Gaithersburg , Maryland , United States )
- Bakhai, Ameet ( Royal Free NHS , London , United Kingdom )
- Bonaca, Marc ( CPC Clinical Research , Aurora , Colorado , United States )
- Chen, Hungta ( Astrazeneca , Wilmington , Delaware , United States )
- Shivappa, Nitin ( Astrazeneca , Wilmington , Delaware , United States )
- Winkelmayer, Wolfgang ( Baylor College of Medicine , Houston , Texas , United States )
- Tangri, Navdeep ( Univ of Manitoba , Winnipeg , Manitoba , Canada )
- Sundin, Anna-karin ( Astrazeneca , Wilmington , Delaware , United States )
- Schneider, Markus P. ( University of Erlangen-Nürnberg , Nürnberg , Germany )
- Bover, Jordi ( Hospital Universitari Germans Trias , Badalona , Spain )
- Fried, Linda ( Univ of Pittsburgh , Pittsburgh , Pennsylvania , United States )
Meeting Info:
Session Info:
At the Crossroads: The Epidemiology of CVD in CKD
Saturday, 11/08/2025 , 09:15AM - 10:05AM
Moderated Digital Poster Session
More abstracts on this topic:
Waseem Neha, Nouman Zainab, Chaudhry Sohaib Aftab Ahmad, Tariq Waleed, Khan Iftikhar, Shah Mazhar, Farooqi Hanzala Ahmed, Faiz Muneeb
Myocardial Protection by Adenosine Triphosphate-Sensitive Potassium Channel Opener Diazoxide Involves Sulfonylurea Receptor (SUR) 2 SubunitWang Jie, Bradshaw Alleabelle, Tryon Robert, Pan Rachel, Holmes Sari, Nichols Colin, Lawton Jennifer
More abstracts from these authors:
Bonaca Marc, Creager Mark
Discussant: CLEAR SYNERGYBonaca Marc