Final ID: MP1735
Promoting the Thrombosis-Induced Alox15 Pathway for the Treatment of Inflammatory Lung Injury
Abstract Body (Do not enter title and authors here): Introduction. There are no effective treatments for sepsis-induced acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). ALI/ARDS patients exhibit increased incidence of thrombosis, but the impact of lung thrombosis on ALI/ARDS is unclear. Innocuous lung thrombosis induces the arachidonate 15-lipoxygenase (Alox15) pathway in lung endothelial cells (ECs) and controls lung EC death. However, the upstream regulators and downstream targets of thrombosis-induced Alox15 are unknown, and the Alox15 pathway has not been targeted for the treatment of ALI/ARDS. Our aim was to define and target the thrombosis-induced endothelial Alox15 pathway for the treatment of ALI/ARDS.
Hypothesis. The endothelial Alox15 pathway inhibits lung endothelial apoptosis and ALI/ARDS.
Methods. In mice, we used multiple models of lung thrombosis and ALI/ARDS, comprehensive lipidomic profiling, and nanoparticle-mediated endothelial gene editing. In humans, we assessed ALI/ARDS lungs by RNA hybridization and co-immunostaining.
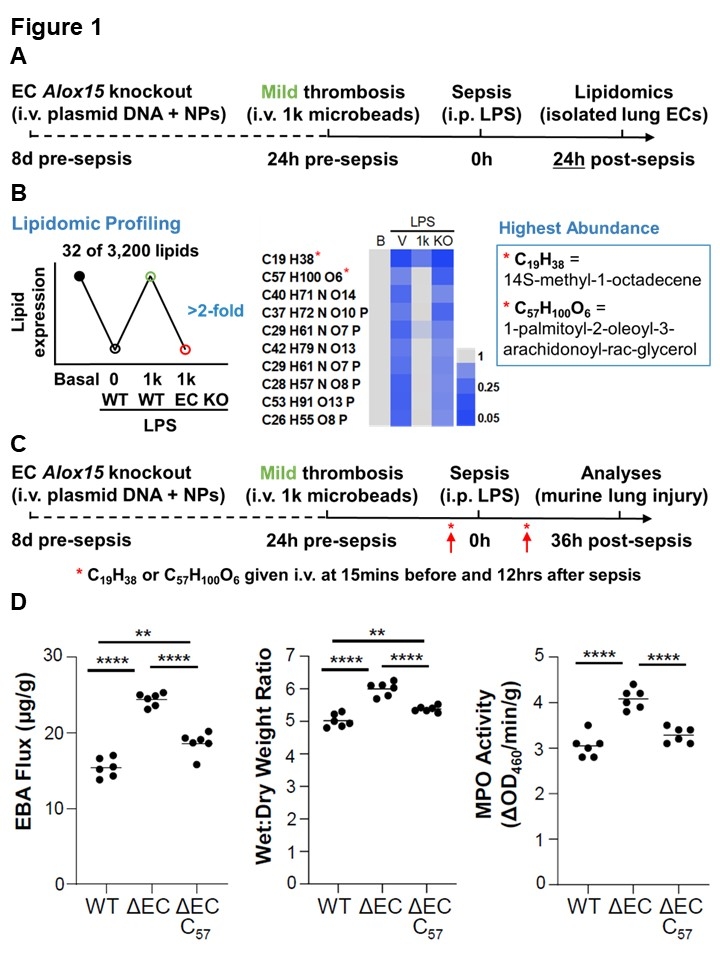
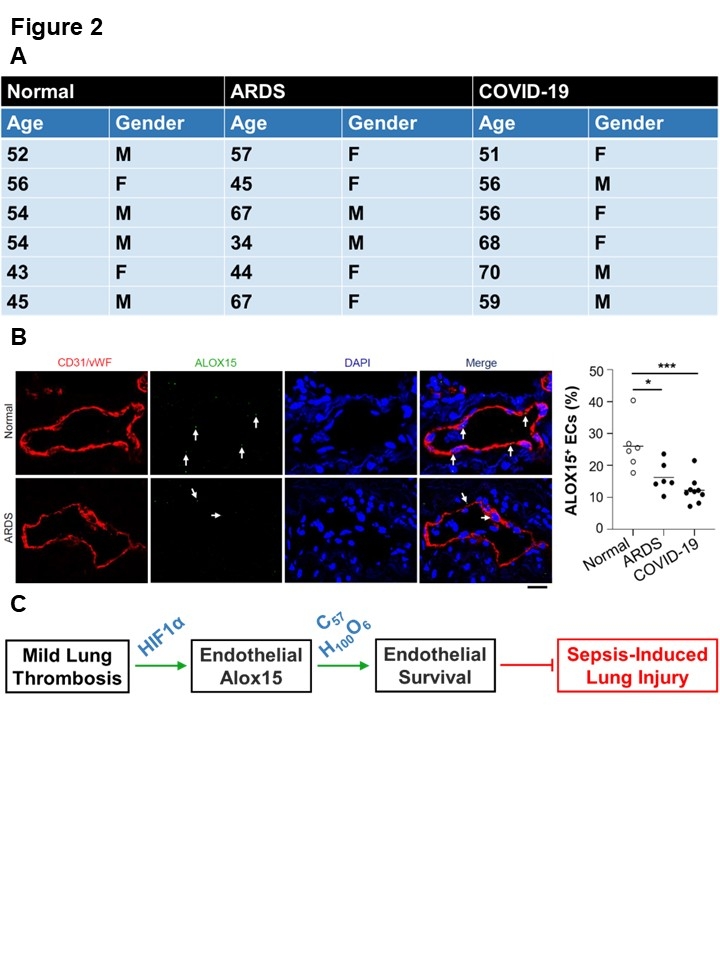
Results. We used nanoparticle-mediated endothelial gene editing to show that endothelial hypoxia-inducible factor 1α regulates Alox15. To determine how endothelial Alox15 mediates the protective impact of innocuous lung thrombosis on ALI/ARDS, we performed lipid profiling of lung ECs from mice with innocuous lung thrombosis and endothelial Alox15 knockout versus controls (Figure 1A). We identified 32 candidate lipids, of which the most abundant were 14S-methyl-1-octadecene (C19H38) and 1-palmitoyl-2-oleoyl-3-arachidonoyl-rac-glycerol (C57H100O6) (Figure 1B). To assess which lipid was responsible for Alox15-dependent inhibition of ALI, we carried out lipid rescue studies (Figure 1C). In sepsis-challenged mice with innocuous lung thrombosis, endothelial Alox15 knockout increased the levels of lung injury and inflammation versus wildtypes; these effects were reversed by treatment with C57H100O6 but not C19H38 (Figure 1D). In human lungs, the proportion of ALOX15+ lung ECs was reduced in ARDS patients compared with normal controls (Figure 2A-B).
Conclusions. Promoting the Alox15 pathway via delivery of C57H100O6 represents a promising therapeutic strategy against ALI/ARDS (Figure 2C).
Hypothesis. The endothelial Alox15 pathway inhibits lung endothelial apoptosis and ALI/ARDS.
Methods. In mice, we used multiple models of lung thrombosis and ALI/ARDS, comprehensive lipidomic profiling, and nanoparticle-mediated endothelial gene editing. In humans, we assessed ALI/ARDS lungs by RNA hybridization and co-immunostaining.
Results. We used nanoparticle-mediated endothelial gene editing to show that endothelial hypoxia-inducible factor 1α regulates Alox15. To determine how endothelial Alox15 mediates the protective impact of innocuous lung thrombosis on ALI/ARDS, we performed lipid profiling of lung ECs from mice with innocuous lung thrombosis and endothelial Alox15 knockout versus controls (Figure 1A). We identified 32 candidate lipids, of which the most abundant were 14S-methyl-1-octadecene (C19H38) and 1-palmitoyl-2-oleoyl-3-arachidonoyl-rac-glycerol (C57H100O6) (Figure 1B). To assess which lipid was responsible for Alox15-dependent inhibition of ALI, we carried out lipid rescue studies (Figure 1C). In sepsis-challenged mice with innocuous lung thrombosis, endothelial Alox15 knockout increased the levels of lung injury and inflammation versus wildtypes; these effects were reversed by treatment with C57H100O6 but not C19H38 (Figure 1D). In human lungs, the proportion of ALOX15+ lung ECs was reduced in ARDS patients compared with normal controls (Figure 2A-B).
Conclusions. Promoting the Alox15 pathway via delivery of C57H100O6 represents a promising therapeutic strategy against ALI/ARDS (Figure 2C).
More abstracts on this topic:
Effects of RAMP expression on location-biased signaling by CLR
Roy Bipradas, Jassal Chanpreet, Viswanathan Gayathri, Nazo Nour, Yu Yen-rei, Rajagopal Sudarshan
84 Immune checkpoint profiling in major aortic diseases leads to identification of potential roles of CD155-CD206 pathway in suppressing inflammation and immune responsesShao Ying, Saaoud Fatma, Xu Keman, Lu Yifan, Jiang Xiaohua, Wang Hong, Yang Xiaofeng