Final ID: MDP747
Semaglutide and cardiovascular outcomes by blood pressure in the SELECT trial
Research Question and Aim: To evaluate the effect of once-weekly semaglutide 2.4 mg vs placebo on cardiovascular (CV) outcomes by baseline BP categories in SELECT.
Methods: SELECT was a double-blind, randomized, placebo-controlled trial that included patients aged ≥45 years with preexisting CV disease and BMI ≥27 kg/m2 without diabetes. Patients received once-weekly semaglutide 2.4 mg or placebo; the primary endpoint was time to first MACE analyzed with a Cox proportional hazards model with semaglutide and placebo as fixed factors according to baseline BP categories.
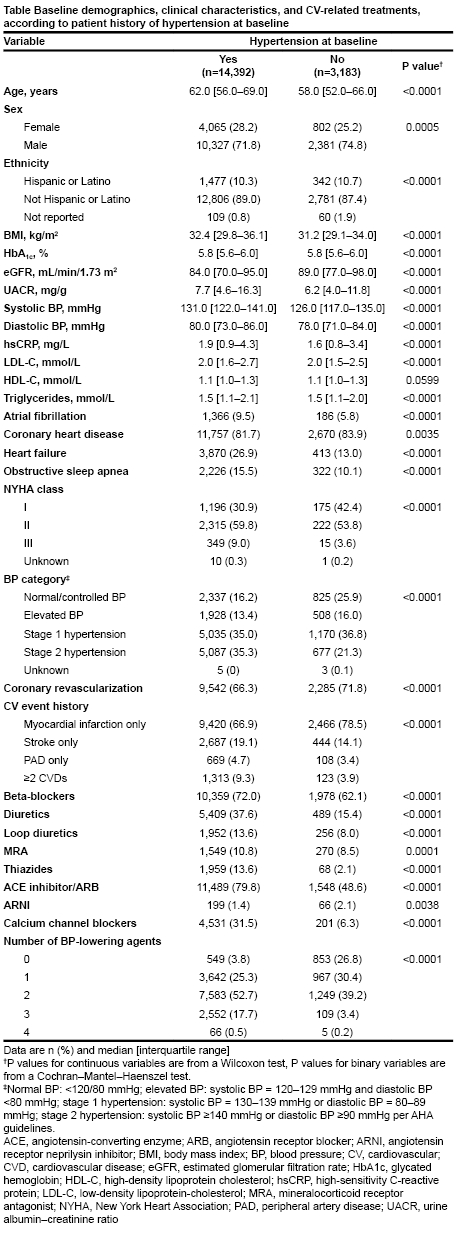
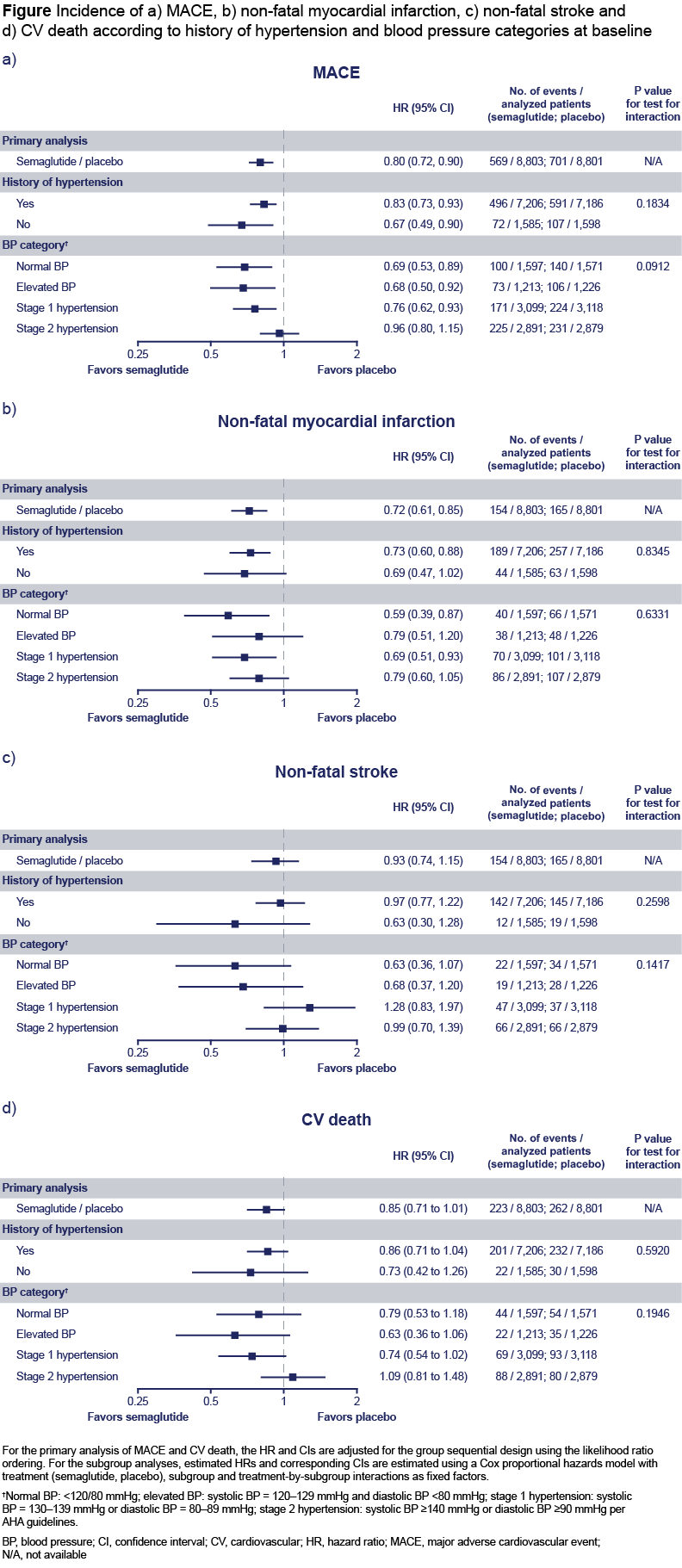
Results: Among 17,604 randomized patients, 14,392 (82%) had a history of HT. Patients with history of HT were older and more likely female, with a higher BMI, lower eGFR, and higher UACR. A higher proportion presented with atrial fibrillation, obstructive sleep apnea, and heart failure NYHA class II/III, but fewer underwent coronary revascularization (Table). Patients with HT who received placebo had a higher incidence rate of MACE vs semaglutide (2.6 vs 2.1 per 100 patient-years, respectively). Semaglutide generally exhibited consistent benefits for CV outcomes regardless of history of HT or baseline BP categories (Figure). Compared with placebo, semaglutide significantly and consistently reduced systolic BP (SBP) across BP categories (normal, −2.9 [95% CI−4.0; −1.9]; elevated BP, −2.8 [−4.0; −1.6]; stage 1, −3.4: [−4.1; −2.6]; stage 2, −3.7 [− 4.4; −2.9]).
Conclusions: HT is highly prevalent in people with overweight or obesity and atherosclerotic CV disease without diabetes and is associated with increased MACE. Semaglutide led to consistent reductions in MACE and lowered SBP irrespective of baseline BP category.
- Verma, Subodh ( St Michael’s Hospital in Toronto and University of Toronto , Toronto , Ontario , Canada )
- Lingvay, Ildiko ( Department of Internal Medicine/Endocrinology and Peter O’ Donnel Jr. School of Public Health, University of Texas Southwestern Medical Center , Dallas , Texas , United States )
- Maeng, Michael ( Department of Cardiology, Aarhus University Hospital, and Department of Clinical Medicine, Aarhus University , Aarhus , Denmark )
- Maher, Vincent ( Trinity College Dublin and Tallaght University Hospital , Dublin , Ireland )
- Parkhomenko, Oleksandr ( National Scientific Centre named after M.D. Strazhesko , Kiev , Ukraine )
- Poulter, Neil ( Imperial Clinical Trials Unit, Imperial College London , London , United Kingdom )
- Van De Borne, Philippe ( Department of Cardiology, Erasme Hospital, Université Libre de Bruxelles , Brussels , Belgium )
- Weeke, Peter ( Novo Nordisk A/S , Copenhagen , Denmark )
- Lincoff, Abraham ( Department of Cardiovascular Medicine, Cleveland Clinic and Cleveland Clinic Lerner College of Medicine of Case Western Reserve University , Cleveland , Ohio , United States )
- Deanfield, John ( Institute of Cardiovascular Science, University College London , London , United Kingdom )
- Arbel, Yaron ( Department of Cardiology, Tel Aviv Sourasky Medical Center, affiliated with the School of Medicine, Tel Aviv University , Tel Aviv , Israel )
- Cariou, Bertrand ( Nantes Université, CHU Nantes, CNRS, INSERM, l’institut du thorax , Nantes , France )
- Matos, Ana Laura S.a. ( Novo Nordisk A/S , Copenhagen , Denmark )
- Hovingh, G. Kees ( Novo Nordisk A/S , Copenhagen , Denmark )
- Jeppesen, Ole Kleist ( Novo Nordisk A/S , Copenhagen , Denmark )
- Kahn, Steven ( Department of Medicine, VA Puget Sound Health Care System and University of Washington, , Seattle , Washington , United States )
- Latkovskis, Gustavs ( Institute of Cardiology and Regenerative Medicine, University of Latvia , Riga , Latvia )
Meeting Info:
Session Info:
Hypertension Gets Exciting! New Drugs! New Technologies! What Should I Choose?
Sunday, 11/17/2024 , 03:15PM - 04:30PM
Moderated Digital Poster Session
More abstracts on this topic:
Nakayama Atsuko, Sakuma Hiroki, Iwata Tomoharu, Kashino Kunio, Isobe Mitsuaki, Tomoike Hitonobu
A major effect of aprocitentan on albuminuria in patients with resistant hypertensionSchlaich Markus, Bakris George, Flack John, Gimona Alberto, Narkiewicz Krzysztof, Sassi-sayadi Mouna, Wang Jiguang, Weber Michael
More abstracts from these authors:
Lingvay Ildiko, Deanfield John, De Los Angeles Quiroga Pelaez Maria, Kahn Steven, Lincoff Abraham, Plutzky Jorge, Ross Stine, Weeke Peter, Ryan Donna
Semaglutide Improves Cardiovascular Outcomes in Patients with a History of Coronary Artery Bypass Surgery and Overweight or Obesity: The SELECT TrialVerma Subodh, Lincoff Abraham, Emerson Scott, Plutzky Jorge, Kahn Steven, Stensen Signe, Weeke Peter, Rasmussen Soren, Poirier Paul, Lingvay Ildiko