Final ID: 4171315
Finerenone and Risk of Hyperkalemia in Patients with Heart failure with Mildly Reduced or Preserved Ejection Fraction
Methods: FINEARTS-HF was a multicenter, randomized, double-blind trial comparing finerenone (titrated to 20 or 40mg, depending on baseline kidney function) versus placebo in patients with HF with LVEF ≥40%, structural heart disease, and elevated natriuretic peptide levels. Per protocol, investigators down-titrated or temporarily interrupted study medication if potassium levels were ≥5.5mmol/l. The primary endpoint was the composite of total worsening HF events and cardiovascular death. Landmark analyses were conducted to examine risks of the primary endpoint subsequent to 1-month measurements of potassium levels separately in each treatment arm.
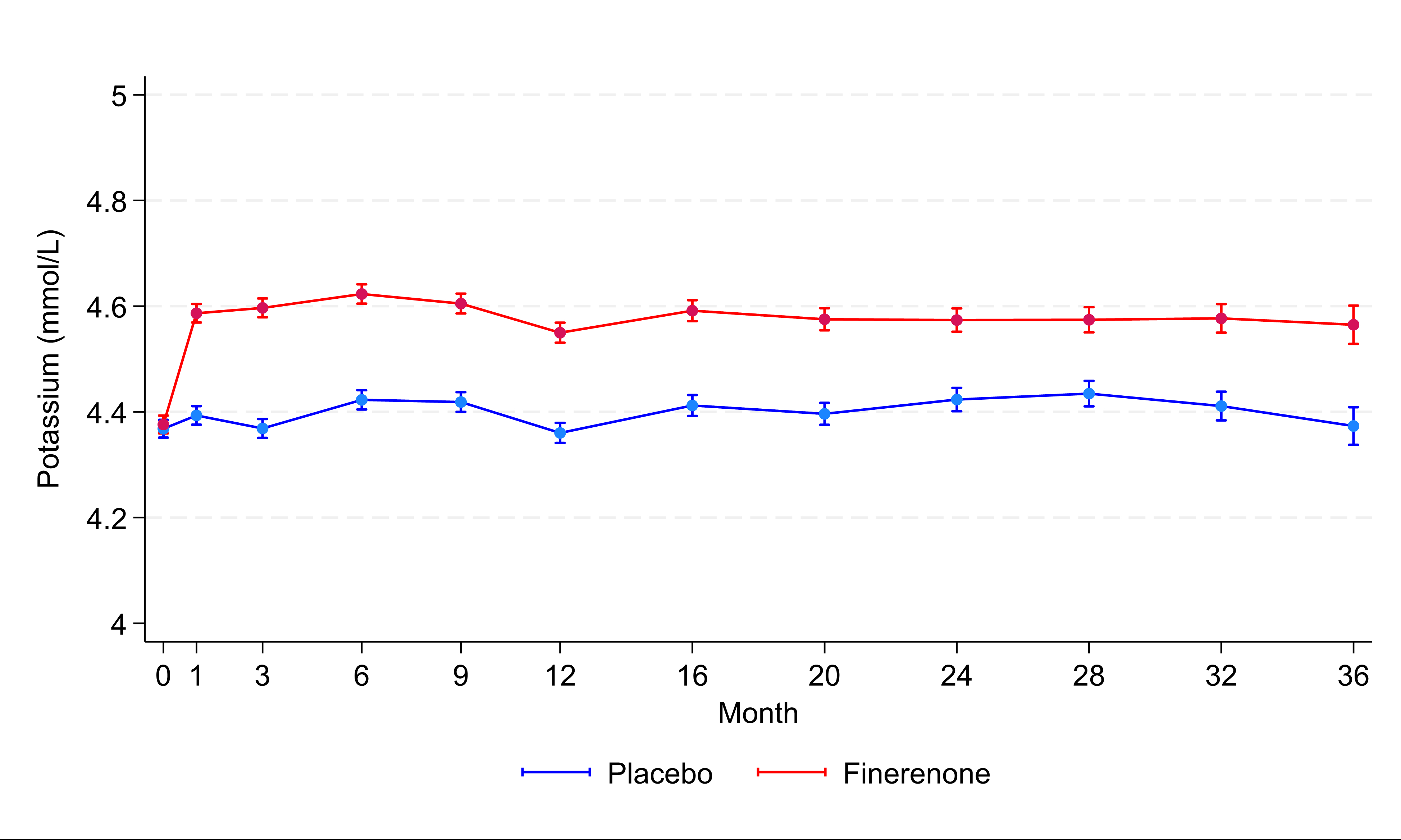
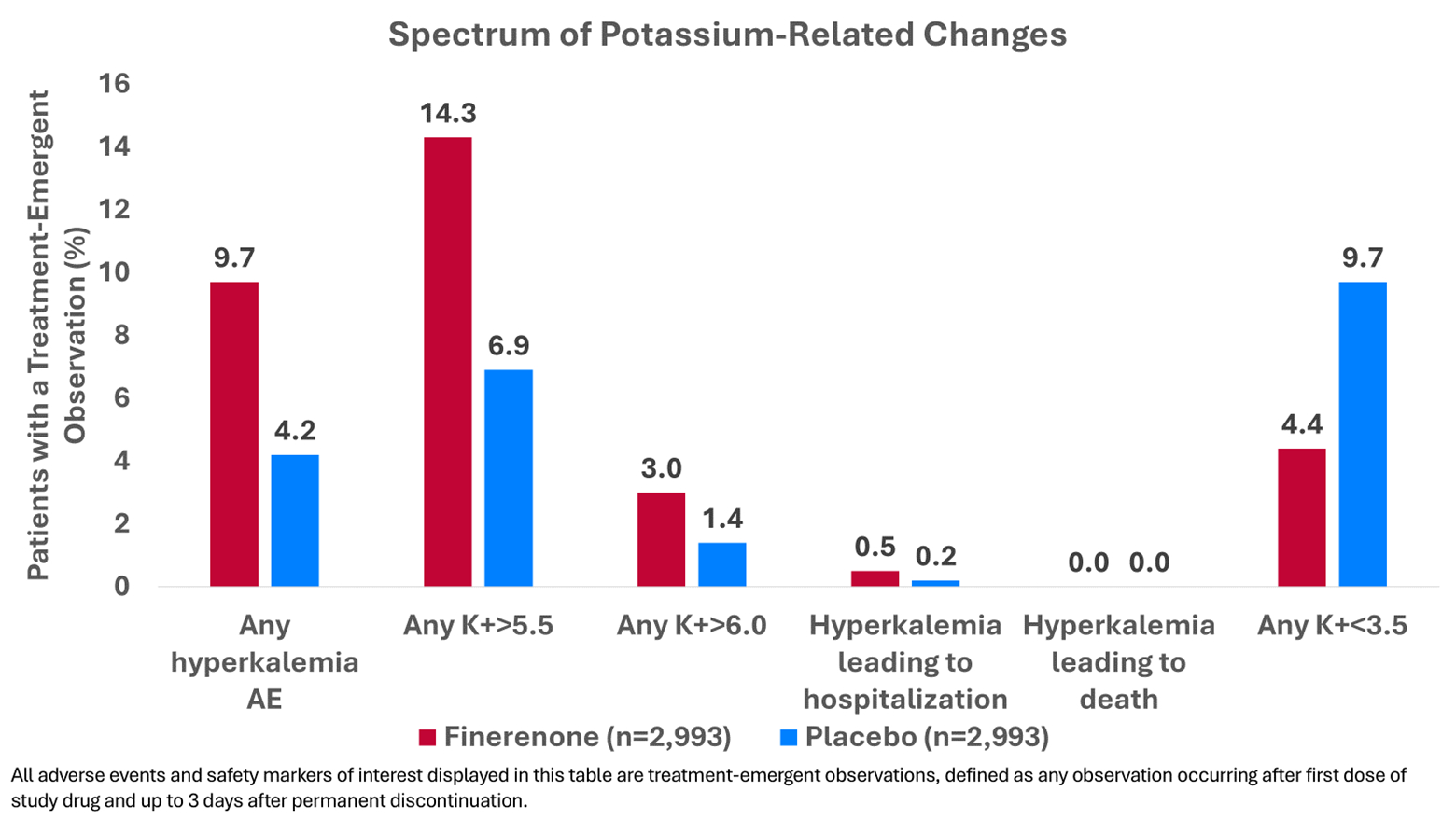
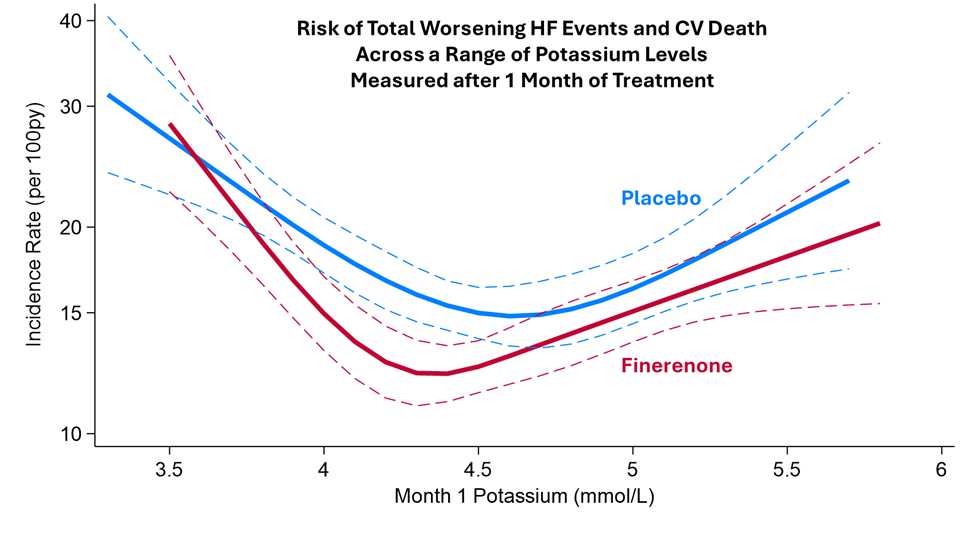
Results: Among 6,001 randomized participants, mean baseline potassium levels were 4.4±0.5mmol/l. At 1 month, the effect of finerenone on potassium was +0.19 (0.17-0.21) mmol/l, a difference that persisted during the trial (Figure 1). Regardless of treatment assignment, patients who developed any potassium level >5.5mmol/l in follow-up were more likely to be men, with histories of diabetes and recent worsening HF events, worse baseline kidney function and higher baseline potassium levels. Finerenone increased the risks of any potassium level >5.5mmol/l (14.3% vs. 6.9%), and decreased the risks for hypokalemia (4.4% vs. 9.7%). Hyperkalemia-related hospitalizations were infrequent, and there were no hyperkalemia-related deaths in either arm (Figure 2). During a median follow-up of 2.6 years, a U-shaped association was observed between potassium levels measured after 1 month of treatment and subsequent risks of the primary outcome, such that for any given level of potassium, risks were generally lower in patients treated with finerenone compared to patients treated with placebo (Figure 3).
Conclusions: In patients with HFmrEF/HFpEF, finerenone resulted in early modest increases in potassium levels. However, the clinical benefit of finerenone was maintained even in the setting of moderate hyperkalemia.
- Vardeny, Orly ( Minneapolis VA Health Care System , Minneapolis , Minnesota , United States )
- Zannad, Faiez ( INSERM-CIC , Vandoeuvre Les Nancy , France )
- Pitt, Bertram ( University of Michigan School , Ann Arbor , Michigan , United States )
- Lay-flurrie, James ( Bayer , Reading , United Kingdom )
- Viswanathan, Prabhakar ( Bayer , Whippany , New Jersey , United States )
- Horvat-broecker, Andrea ( Bayer , Wuppertal , Germany )
- Scalise, Andrea ( Bayer , Barcelona , Spain )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Jhund, Pardeep ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Lam, Carolyn ( NATIONAL HEART CENTRE SINGAPORE , Singapore , Singapore )
- Senni, Michele ( ASST PAPA GIOVANNI XXIII , BERGAMO , Italy )
- Shah, Sanjiv ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Voors, Adriaan ( UNIVERSITY MEDICAL CENTER GRONINGEN , Gronien , Netherlands )
Meeting Info:
Session Info:
Featured Science: Getting Closer to the Summit: New HFpEF Treatments
Sunday, 11/17/2024 , 08:00AM - 09:15AM
Featured Science
More abstracts on this topic:
Adhikari Ashok, Tadigotla Chandana, Gaddam Ashwith Reddy, Vaghamashi Yogeshkumar, Gupta Umang, Meda Venkata Sai Abhilash, Panjiyar Binay
Mineralocorticoid receptor antagonist in patients with acute myocardial infarction: An updated systematic review and meta-analysis of randomized trialsD'entremont Marc-andre, Montalescot Gilles, Zannad Faiez, Beygui Farzin, Pitt Bertram, Jolly Sanjit, Cheema Zain, Chauhan Ashwin, Kedev Sasko, Cornel Jan, Stankovic Goran, Moreno Raul, Storey Robert, Bossard Matthias
More abstracts from these authors:
Kondo Toru, Amarante Flaviana, Lam Carolyn, Senni Michele, Shah Sanjiv, Voors Adriaan, Zannad Faiez, Pitt Bertram, Vaduganathan Muthiah, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Claggett Brian, Desai Akshay, Brinker Meike, Lay-flurrie James, Schloemer Patrick, Viswanathan Prabhakar
Race does not influence the efficacy and safety of mineralocorticoid-receptor antagonists in heart failure: An individual-participant data meta-analysis of 4 trialsButt Jawad, Pitt Bertram, Senni Michele, Shah Sanjiv, Zannad Faiez, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Talebi Atefeh, Vardeny Orly, Claggett Brian, Vaduganathan Muthiah, Desai Akshay, Lam Carolyn