Final ID: 4170814
Efficacy and safety of finerenone in patients with heart failure and mildly reduced or preserved ejection fraction: A prespecified sex-specific analysis of the FINEARTS-HF trial
Methods: FINEARTS-HF was a randomized, double-blind, multicenter, event-driven, trial in patients with left ventricular ejection fraction ≥40%, investigating the efficacy and safety of the non-steroidal mineralocorticoid receptor antagonist finerenone, compared to placebo, in patients with HF and mildly reduced or preserved ejection fraction (HFmrEF/HFpEF). The primary outcome was the composite of cardiovascular death and total (first and repeat) HF events (either an unplanned HF hospitalization or an urgent HF visit). In a prespecified subgroup analysis, we evaluated the efficacy and safety of finerenone compared to placebo in both women and men.
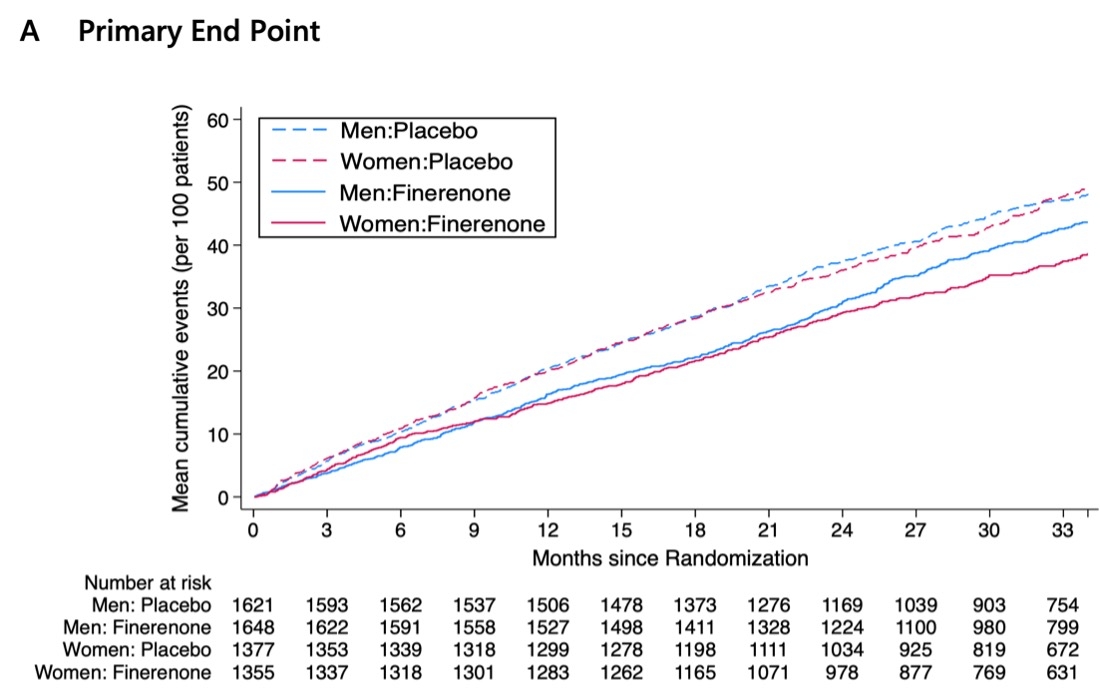
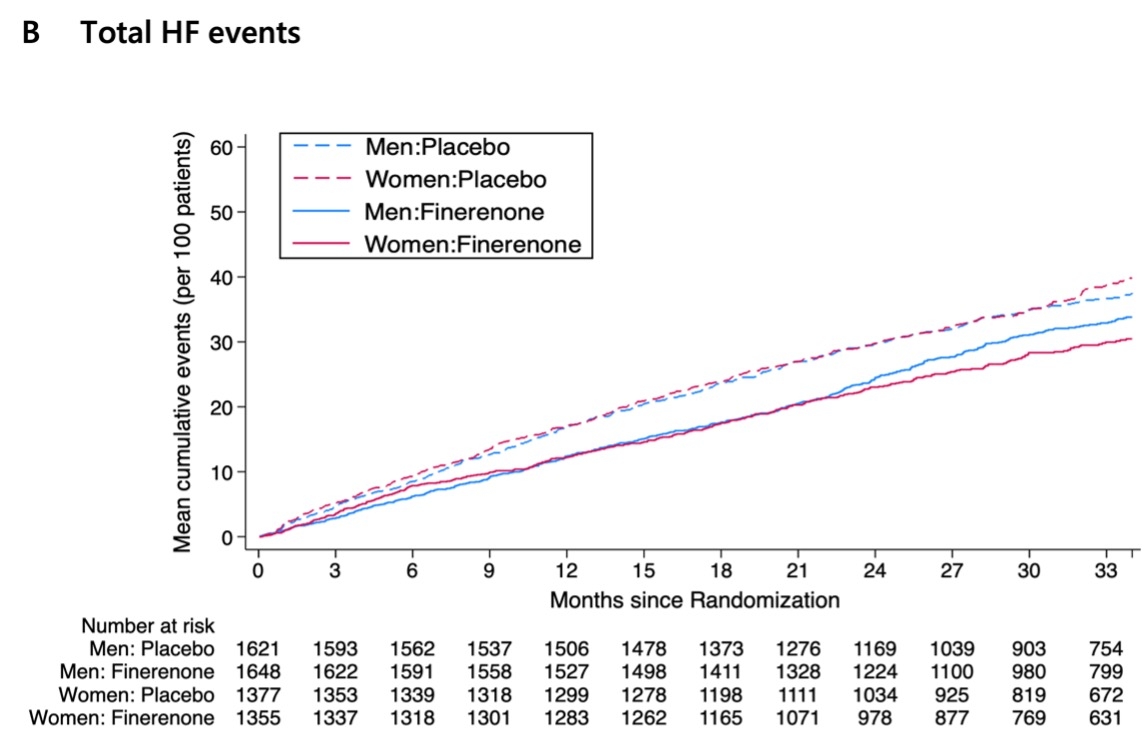
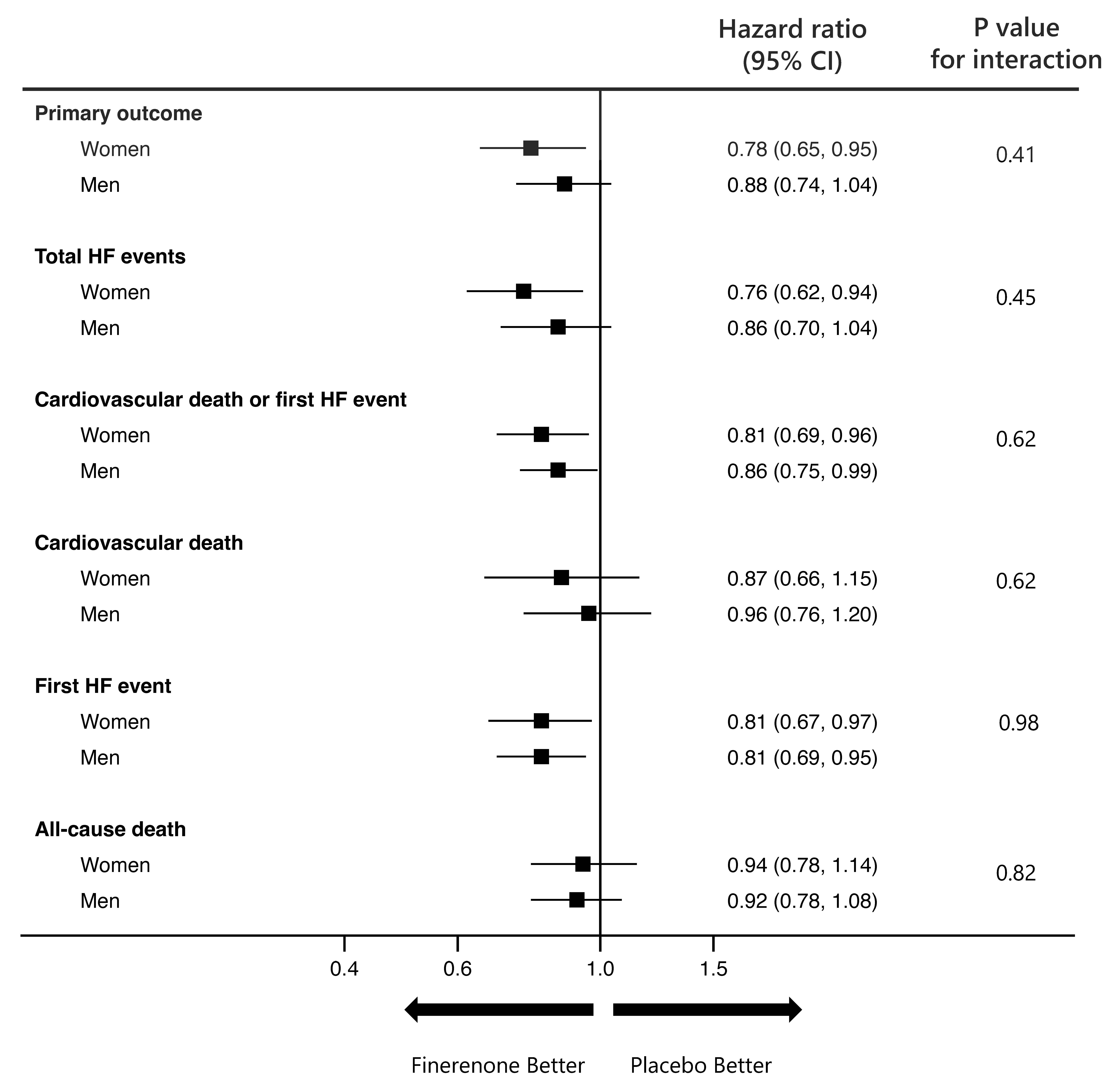
Results: Overall, 2,732 women (45.5%) and 3,269 men (54.5%) were analyzed. Women were older than men and had higher rates of obesity, lower estimated glomerular filtration rate, worse NYHA functional class, and lower Kansas City Cardiomyopathy Questionnaire-total symptom scores (KCCQ-TSS). The rate of the primary outcome in the placebo group was similar between women and men. Compared to placebo, finerenone reduced the risk of the primary outcome to a similar extent in both women and men, with rate ratios (RRs) of 0.78 (95%CI 0.65-0.95) in women versus 0.88 (0.74-1.04) in men, respectively (P for interaction = 0.41). Consistent benefits were observed for the components of the primary outcome and all-cause death (Figures 1 and 2). The mean increase (improvement) in KCCQ-TSS from baseline to 12 months was greater with finerenone, irrespective of sex (P for interaction=0.73). Adverse events of interest were similar in both women and men.
Conclusion: In FINEARTS-HF, finerenone was efficacious and safe, irrespective of sex, in patients with HFmrEF/HFpEF.
- Chimura, Misato ( University of glasgow , Glasgow , United Kingdom )
- Lam, Carolyn ( NATIONAL HEART CENTRE SINGAPORE , Singapore , Singapore )
- Senni, Michele ( ASST PAPA GIOVANNI XXIII , BERGAMO , Italy )
- Shah, Sanjiv ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Voors, Adriaan ( UNIVERSITY MEDICAL CENTER GRONINGEN , Gronien , Netherlands )
- Zannad, Faiez ( CVCT and Universite de Lorraine , Paris , France )
- Pitt, Bertram ( University of Michigan School , Ann Arbor , Michigan , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Mcmurray, John ( University of glasgow , Glasgow , United Kingdom )
- Jhund, Pardeep ( University of glasgow , Glasgow , United Kingdom )
- Henderson, Alasdair David ( University of glasgow , Glasgow , United Kingdom )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Mueller, Katharina ( Bayer AG , Berlin , Germany )
- Glasauer, Andrea ( Bayer AG , Berlin , Germany )
- Rohwedder, Katja ( Bayer AG , Berlin , Germany )
- Viswanathan, Prabhakar ( Bayer AG , Berlin , Germany )
Meeting Info:
Session Info:
Featured Science: Getting Closer to the Summit: New HFpEF Treatments
Sunday, 11/17/2024 , 08:00AM - 09:15AM
Featured Science
More abstracts on this topic:
Liu Olivia, Li Yiwei, Reynolds Harmony, Spruill Tanya, Arabadjian Milla
Mineralocorticoid receptor antagonist in patients with acute myocardial infarction: An updated systematic review and meta-analysis of randomized trialsD'entremont Marc-andre, Montalescot Gilles, Zannad Faiez, Beygui Farzin, Pitt Bertram, Jolly Sanjit, Cheema Zain, Chauhan Ashwin, Kedev Sasko, Cornel Jan, Stankovic Goran, Moreno Raul, Storey Robert, Bossard Matthias
More abstracts from these authors:
Kondo Toru, Amarante Flaviana, Lam Carolyn, Senni Michele, Shah Sanjiv, Voors Adriaan, Zannad Faiez, Pitt Bertram, Vaduganathan Muthiah, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Claggett Brian, Desai Akshay, Brinker Meike, Lay-flurrie James, Schloemer Patrick, Viswanathan Prabhakar
Efficacy of finerenone in patients with heart failure and mildly reduced or preserved ejection fraction: A prespecified analysis of heart rate in the FINEARTS-HF trialChimura Misato, Senni Michele, Zannad Faiez, Pitt Bertram, Vaduganathan Muthiah, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Claggett Brian, Desai Akshay, Lay-flurrie James, Scalise Andrea, Rohwedder Katja, Lam Carolyn