Final ID: 4160207
Pragmatic Randomized Controlled Trial of a Decision Support System to Aid Physician Optimization of Early Lipid Lowering Therapies after Acute Coronary Syndrome
Patients with acute coronary syndromes (ACS) are at high risk of recurrent cardiovascular disease (CVD) events. Most are not on lipid-lowering therapy (LLT) at admission and LLT is typically initiated before discharge. Under current standard-of-care (SoC) pathways, additional LLT adjustments are often needed over months or years post-ACS to achieve low-density lipoprotein cholesterol (LDL-C) goals. This stepwise optimization relies solely on achieved LDL-C levels rather than estimated CVD risk reductions from further LDL-C lowering through adjunctive LLT or intensified statin monotherapy.
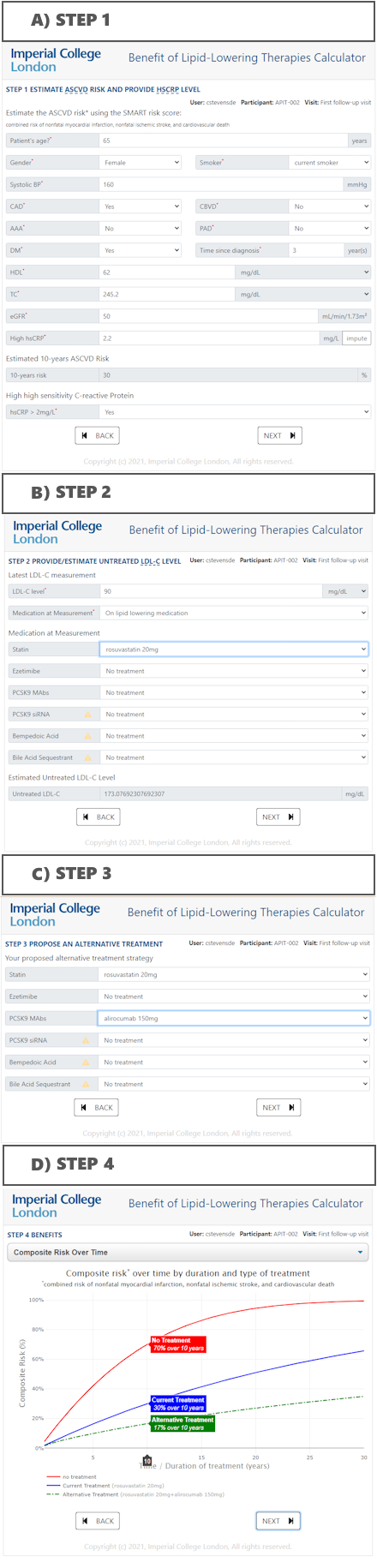
We hypothesized that a Decision Support System (DSS) providing information on residual CVD risk and potential benefits of various LLTs may lead to earlier optimization compared to SoC (Figure 1). If confirmed, integrating a pragmatic DSS into electronic health records could accelerate LDL-C goal achievement and improve patient outcomes.
Study Design and Methods
An interventional, parallel-assignment, statistician-blinded, cluster-randomized controlled trial with a 16-week follow-up. The primary endpoint will be analyzed with a generalized linear mixed model (GLM) with fixed effects for country and site type and a random effect for study site. The secondary endpoints will be analyzed using Kaplan-Meier plots, Cox proportional hazards and GLM models.
Sample Size
1140 patients from 42 sites in the UK, Italy, and Spain, randomized to the DSS or SoC (tabulated in Figure 2).
Population Studied
Adults <80 years hospitalized ≤72 hours post-ACS.
Intervention
A DSS offering clinicians patient-specific absolute 10-year risk of atherosclerotic CVD and potential risk reductions over time for different LLT regimens and combinations (Fig. 1).
Power Calculations
Up to 48 clusters of 33 participants each are needed to detect a 10% higher use of combination LLT in the DSS group vs the SoC group (15% vs 5%) within 4 months post-ACS (End of Study), with 90% power and an intraclass correlation coefficient of 0.1.
Primary End Point
Proportion of patients undergoing intensified monotherapy or initiating/escalating combination LLT in DSS vs SoC.
Secondary End Points
Time to intensification/escalation of LLT and differences in LDL-C levels and goal achievement.
Outcomes
Baseline patient characteristics in each arm are available in Fig. 2. The primary and secondary endpoints will be analyzed and presented.
Funding
This work from Imperial College London is supported by Sanofi Winthrop Industrie.
- Stevens, Christophe ( Imperial College London , London , United Kingdom )
- Cornelius, Victoria ( Imperial College London , London , United Kingdom )
- Kiru, Gaia ( Imperial College London , London , United Kingdom )
- Janani, Leila ( Imperial College London , London , United Kingdom )
- Zambon, Alberto ( University of Padua , Padua , Italy )
- Lopez-sendon, Jose ( IdiPaz Research Institute , Madrid , Spain )
- Connolly, Derek ( Birmingham City Hospital , Birmingham , United Kingdom )
- Ray, Kausik ( Imperial College London , London , United Kingdom )
- Smith, Jessica ( Imperial College London , London , United Kingdom )
- Brandts, Julia ( RWTH University Hospital Aachen , Aachen , Germany )
- Barkas, Fotis ( University of Ioannina , Ioannina , Greece )
- Lorna, Hazell ( Imperial College London , London , United Kingdom )
- Moreno Morales, Maria ( Imperial College London , London , United Kingdom )
- Khunti, Kamlesh ( University of Leicester , Leicester , United Kingdom )
- Mcevoy, John ( University of Galway , Galway , Ireland )
- Poulter, Neil ( Imperial College London , London , United Kingdom )
Meeting Info:
Session Info:
Innovation in Prevention and Global Implementation
Sunday, 11/17/2024 , 03:30PM - 04:45PM
Late-Breaking Science
More abstracts on this topic:
Rimskaya Elena, Chmelevsky Mikhail, Aparina Olga, Bazhutina Anastasia, Budanova Margarita, Khamzin Svyatoslav, Stukalova Olga, Ternovoy Sergey, Golitsyn Sergey
Closing The CHD Diagnostic Gap: AI-Enhanced Discovery of Disease-Causal Genetic VariantsGaither Jeffrey, Moreland Blythe, Garg Vidu, White Peter
More abstracts from these authors:
Mcevoy John, Muntner Paul
Can Machine Learning Help Prioritise Who to Screen for Elevated Lipoprotein(a) (Lp[a]) in the General Population vs a Screen all Approach? An Analysis from UK BiobankStevens Christophe, Barkas Fotis, Brandts Julia, Kwilasi Sunganani, Mahani Alireza, Vallejo-vaz Antonio J., Taghavi Azar Sharabiani Mansour, Ray Kausik