Final ID: Mo4104
A Rare Case of Sequential Impella Mechanical Failures due to Infective Endocarditis Vegetations
Abstract Body (Do not enter title and authors here): Description of Case:
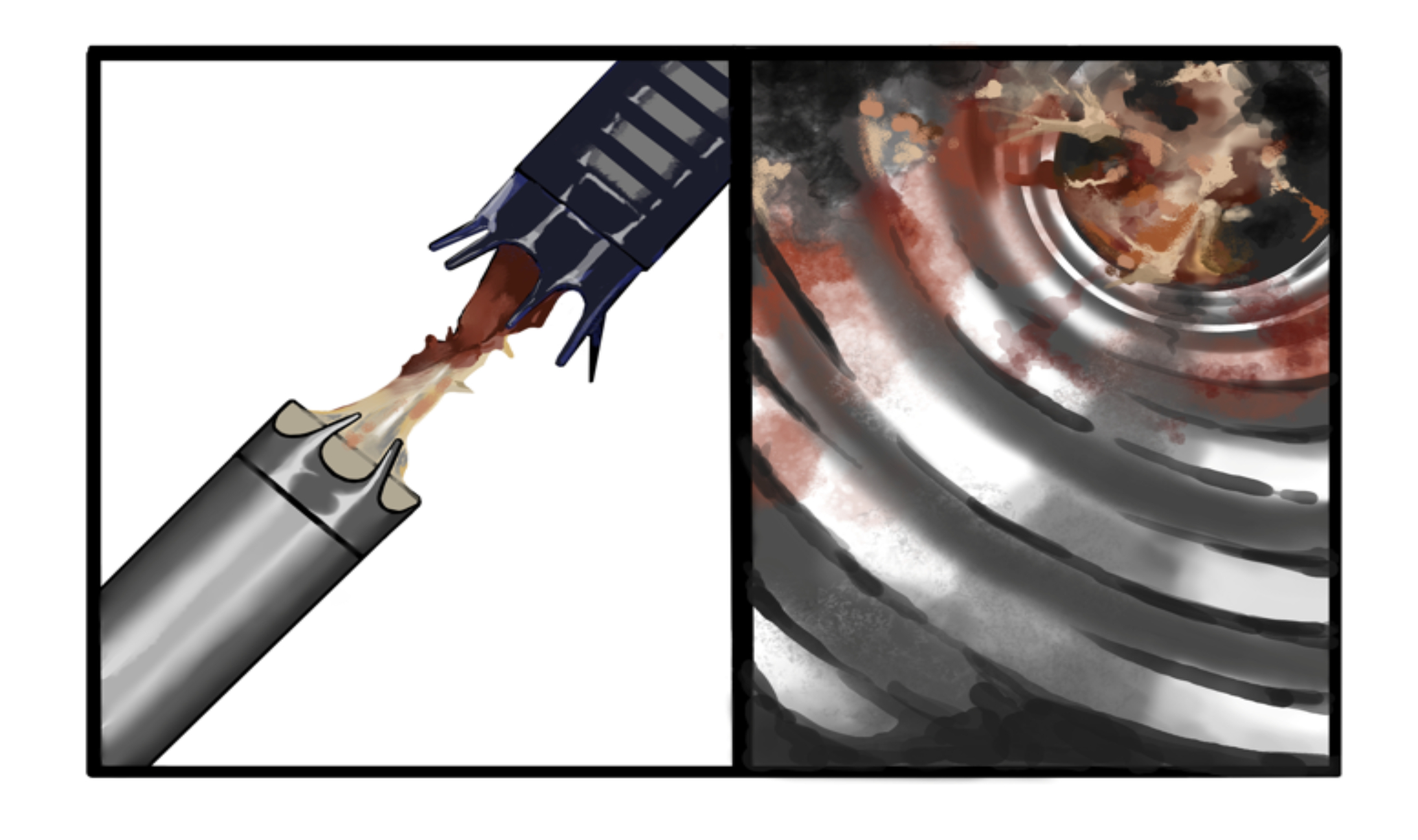
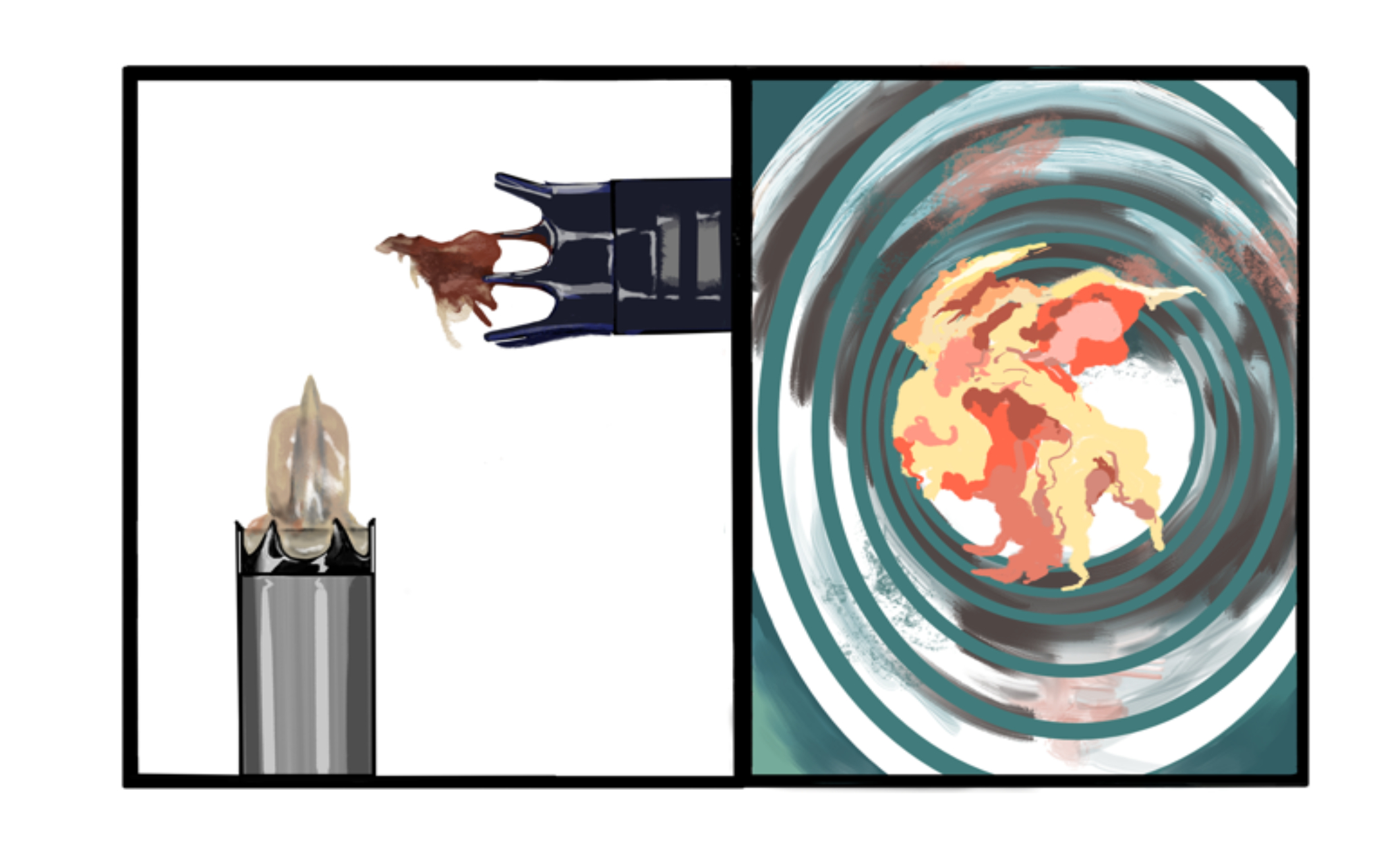
A 64-year-old male with complex medical history, including infective endocarditis of the aortic valve requiring surgical replacement with a bioprosthetic valve and recurrent infective endocarditis of the bioprosthetic valve, presented with two hours of crushing chest pain and found to have ST elevations. Urgent angiography revealed complete occlusion with thrombus of the proximal left anterior descending (LAD) coronary artery. The patient underwent aspiration thrombectomy, followed by intracoronary vasodilators, without improvement of flow. Due to ongoing shock despite initial mechanical support, the patient was escalated to an Impella CP device after a transthoracic echo confirmed no left ventricle thrombus. Once stabilized, intravascular ultrasound showed significant thrombus and plaque in the LAD. This was treated with a drug-eluting stent, but TIMI 3 flow was not achieved. The patient was placed on an integrilin drip with plans to reevaluate in 24 hours. While preparing for transport to the cardiac ICU, the Impella device malfunctioned, and function could not be restored. With ongoing hemodynamic collapse, a second Impella device was placed, but it malfunctioned almost immediately, and the patient suffered a pulseless electrical activity arrest. Advanced Cardiac Life Support was initiated, but the patient ultimately expired. Abiomed's investigation following the case identified an unknown biological material obstructing both Impella devices. In light of a leukocytosis of 14,000 and a recent urine sample positive for E. coli, there is a high suspicion that the patient suffered from recurrent infective endocarditis causing septic embolization and subsequent Impella device failure from aspiration of bacterial vegetations.
Discussion:
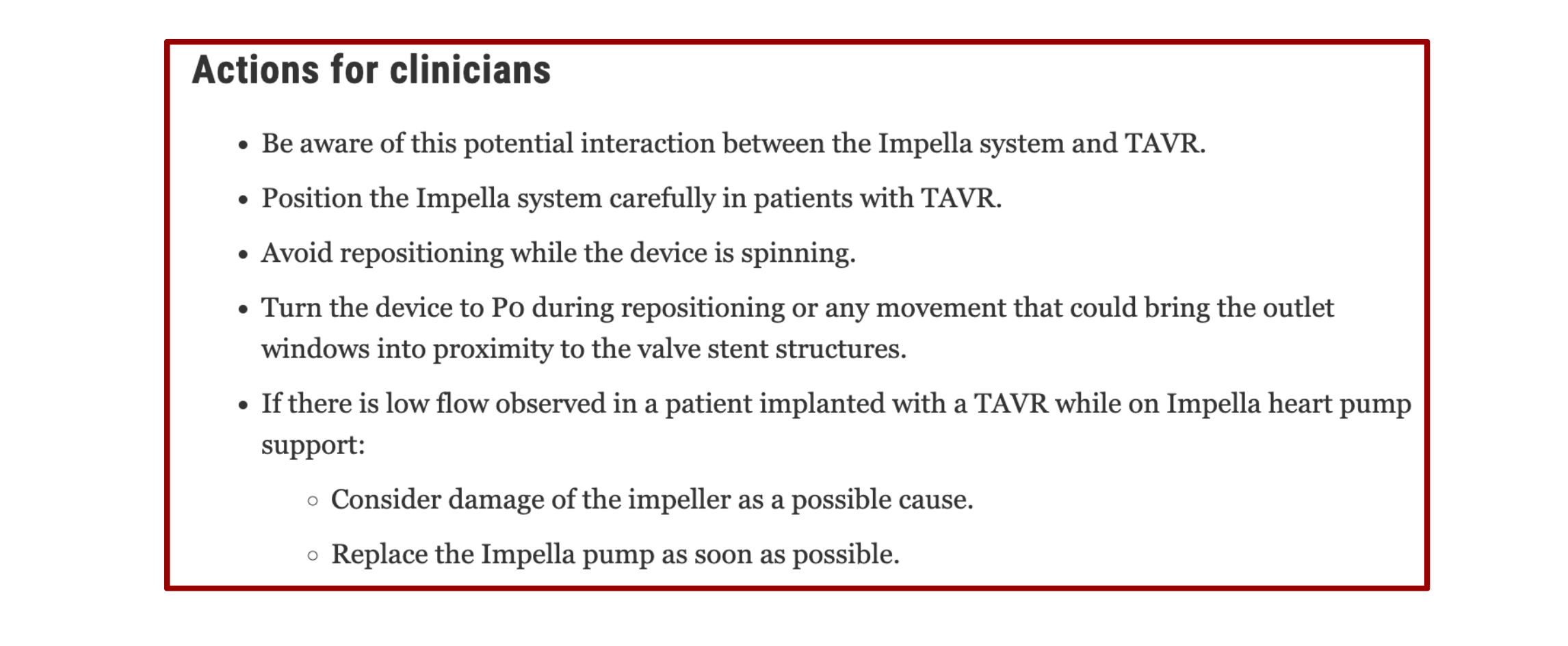
This is the first known reported case of Impella device failure resulting from the aspiration of vegetation in a patient with infective endocarditis of a bioprosthetic aortic valve. The case highlights the challenges of diagnosing endocarditis in bioprosthetic valves and emphasizes the importance of transesophageal echo in cases with high clinical suspicion. Additionally, considering the recent FDA recall of Impella devices in patients who have had transcatheter aortic valve replacement (TAVR) procedures due to the risk of motor damage after contact with TAVR stents, this case calls attention to a need for further research of the use and safety of Impella devices in patients with non-native aortic valves.
A 64-year-old male with complex medical history, including infective endocarditis of the aortic valve requiring surgical replacement with a bioprosthetic valve and recurrent infective endocarditis of the bioprosthetic valve, presented with two hours of crushing chest pain and found to have ST elevations. Urgent angiography revealed complete occlusion with thrombus of the proximal left anterior descending (LAD) coronary artery. The patient underwent aspiration thrombectomy, followed by intracoronary vasodilators, without improvement of flow. Due to ongoing shock despite initial mechanical support, the patient was escalated to an Impella CP device after a transthoracic echo confirmed no left ventricle thrombus. Once stabilized, intravascular ultrasound showed significant thrombus and plaque in the LAD. This was treated with a drug-eluting stent, but TIMI 3 flow was not achieved. The patient was placed on an integrilin drip with plans to reevaluate in 24 hours. While preparing for transport to the cardiac ICU, the Impella device malfunctioned, and function could not be restored. With ongoing hemodynamic collapse, a second Impella device was placed, but it malfunctioned almost immediately, and the patient suffered a pulseless electrical activity arrest. Advanced Cardiac Life Support was initiated, but the patient ultimately expired. Abiomed's investigation following the case identified an unknown biological material obstructing both Impella devices. In light of a leukocytosis of 14,000 and a recent urine sample positive for E. coli, there is a high suspicion that the patient suffered from recurrent infective endocarditis causing septic embolization and subsequent Impella device failure from aspiration of bacterial vegetations.
Discussion:
This is the first known reported case of Impella device failure resulting from the aspiration of vegetation in a patient with infective endocarditis of a bioprosthetic aortic valve. The case highlights the challenges of diagnosing endocarditis in bioprosthetic valves and emphasizes the importance of transesophageal echo in cases with high clinical suspicion. Additionally, considering the recent FDA recall of Impella devices in patients who have had transcatheter aortic valve replacement (TAVR) procedures due to the risk of motor damage after contact with TAVR stents, this case calls attention to a need for further research of the use and safety of Impella devices in patients with non-native aortic valves.
More abstracts on this topic:
An Unusual Case of Listeria LVAD Infection Complicated by Intracranial Catastrophe
Zviman Julieann, Mouradjian Mallory, Rovelli Richard, Leventhal Sarah, Ananthram Manjula, Ramani Gautam, Jones Niya, Griffith Bartley, Dees Lynn, Nevin Amanda, Crowell Leigha, Soares Cullen, Amoroso Anthony
Abatacept Drug-Induced Loeffler Endocarditis: A Manifestation of Hypereosinophilic SyndromeSweeting Alexander, Atalay Michael, Agarwal Saurabh, Hulten Edward, Patel Yash