Final ID: 4147504
Efficacy and Safety of Vorapaxar in Patients with Peripheral Arterial Disease: A Meta-Analysis of Randomized Controlled Trials
Abstract Body (Do not enter title and authors here): Introduction: Vorapaxar is an inhibitor of protease-activated receptor-1 (PAR1) and primarily blocks thrombin mediated platelet activation. There are discrepancies in the literature regarding whether vorapaxar alleviates ischemic outcomes and increases bleeding in patients with peripheral arterial disease (PAD).
Purpose: The aim of this study is to evaluate the efficacy and safety of Vorapaxar in patients with PAD.
Methods: A comprehensive literature search of Cochrane CENTRAL, PubMed, Ovid Medline, and Web of Science databases was conducted. Randomized controlled trials (RCTs) comparing Vorapaxar in patients with PAD were included. Primary efficacy outcome was defined as hospitalization for acute limb ischemia, while primary safety outcome was assessed for severe bleeding events according to GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) trial. Summary effect measures of the primary outcomes were obtained by pooling the data with an inverse variance–weighted random-effects model. Statistical analyses were performed with “meta” package in R (version 4.3.2).
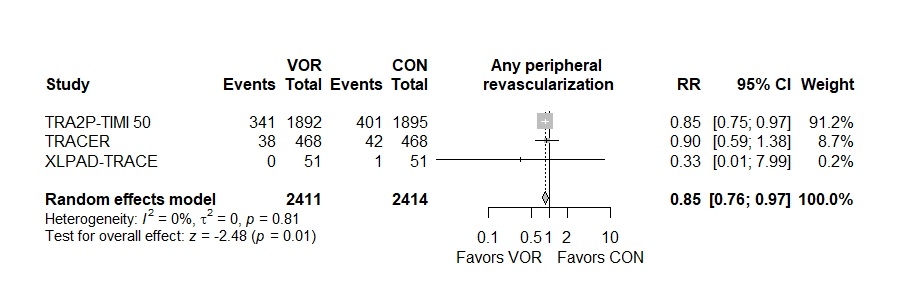
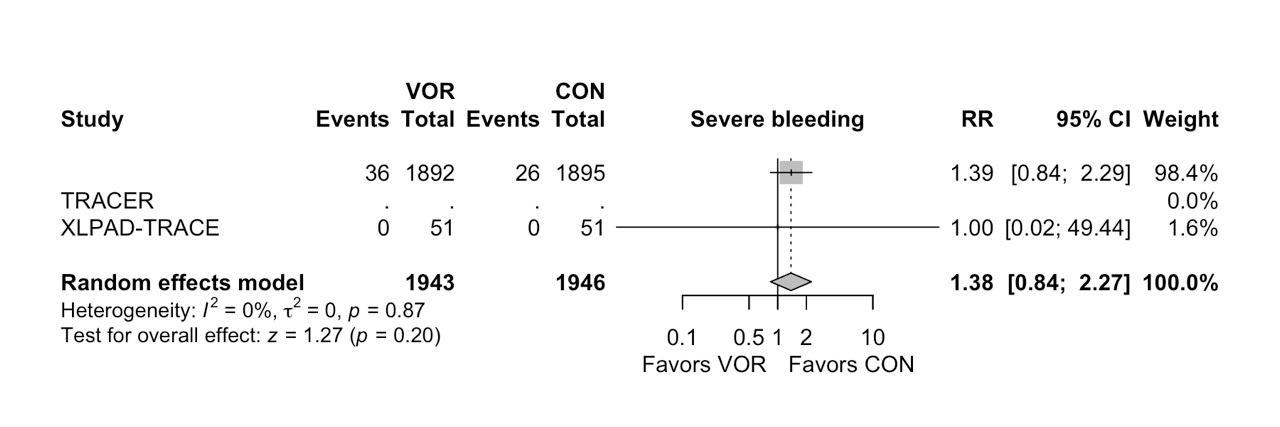
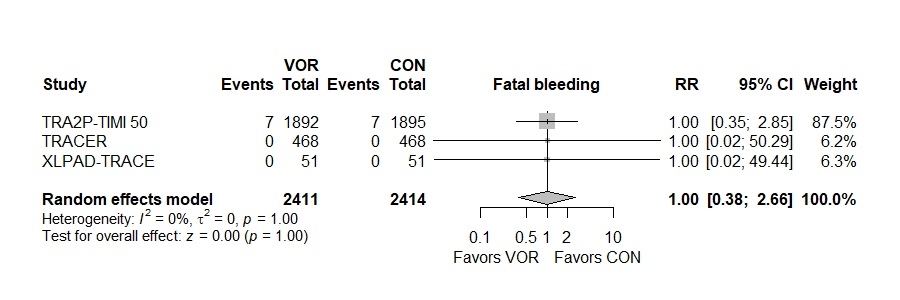
Results: Three unique RCTs involving 4825 patients were included in the analysis. Vorapaxar was associated with a significantly less rate of hospitalization for acute limb ischemia (Risk Ratio [RR] 0.57, 95% Confidence Interval (CI) 0.39 to 0.83, I2: 0%) and significantly less requirement of peripheral revascularization (RR 0.85, 95% CI 0.76 to 0.97, I2: 0%) compared to placebo. Furthermore, no significant differences were observed between Vorapaxar and placebo groups in fatal bleeding (RR 1.00, 95% CI 0.38 to 2.66, I2: 0), severe bleeding according to GUSTO trial (RR 1.38, 95% CI 0.84 to 2.27, I2: 0%), and lower extremity amputation (RR 0.53, 95% CI 0.17 to 1.67, I2: 0%).
Conclusion: In patients with PAD, Vorapaxar significantly reduces hospitalizations due to acute limb ischemia and the need for peripheral revascularizations compared to placebo. However, it does not have a significant effect on the need for lower extremity amputations. In terms of safety, Vorapaxar does not lead to fatal or severe bleedings compared to placebo. Therefore, Vorapaxar should remain one of the treatment options for patients with PAD.
Purpose: The aim of this study is to evaluate the efficacy and safety of Vorapaxar in patients with PAD.
Methods: A comprehensive literature search of Cochrane CENTRAL, PubMed, Ovid Medline, and Web of Science databases was conducted. Randomized controlled trials (RCTs) comparing Vorapaxar in patients with PAD were included. Primary efficacy outcome was defined as hospitalization for acute limb ischemia, while primary safety outcome was assessed for severe bleeding events according to GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) trial. Summary effect measures of the primary outcomes were obtained by pooling the data with an inverse variance–weighted random-effects model. Statistical analyses were performed with “meta” package in R (version 4.3.2).
Results: Three unique RCTs involving 4825 patients were included in the analysis. Vorapaxar was associated with a significantly less rate of hospitalization for acute limb ischemia (Risk Ratio [RR] 0.57, 95% Confidence Interval (CI) 0.39 to 0.83, I2: 0%) and significantly less requirement of peripheral revascularization (RR 0.85, 95% CI 0.76 to 0.97, I2: 0%) compared to placebo. Furthermore, no significant differences were observed between Vorapaxar and placebo groups in fatal bleeding (RR 1.00, 95% CI 0.38 to 2.66, I2: 0), severe bleeding according to GUSTO trial (RR 1.38, 95% CI 0.84 to 2.27, I2: 0%), and lower extremity amputation (RR 0.53, 95% CI 0.17 to 1.67, I2: 0%).
Conclusion: In patients with PAD, Vorapaxar significantly reduces hospitalizations due to acute limb ischemia and the need for peripheral revascularizations compared to placebo. However, it does not have a significant effect on the need for lower extremity amputations. In terms of safety, Vorapaxar does not lead to fatal or severe bleedings compared to placebo. Therefore, Vorapaxar should remain one of the treatment options for patients with PAD.
More abstracts on this topic:
A murine model of cardiovascular-kidney-metabolic syndrome demonstrates compromised limb function in the ischemic hind limb
Lotfollahzadeh Saran, Siracuse Jeffrey, Cabral Howard, Malikova Marina, Sayed Nazish, Chitalia Vipul
Antithrombotic Strategies and Outcomes in Neonates and Infants with Cardiac Shunts: A Systematic Review and Meta-analysisKiskaddon Amy, Do Nhue, Goldenberg Neil, Betensky Marisol, Branstetter Joshua, Ashour Dina, Williams Pamela, Stock Arabela, Silvey Michael, Giglia Therese