Final ID: Mo1038
Apabetalone: evaluating cardiovascular and safety outcomes in meta-analysis
Abstract Body (Do not enter title and authors here): Background: Epigenetic mechanisms play a pivotal role in the transcriptional programs associated with cardiovascular disease (CVD). Apabetalone, an orally available inhibitor of BET proteins that serve as key epigenetic readers, and modulating gene expression - holds promise as a therapeutic intervention in CVD management. Understanding its efficacy and tolerability is crucial for delineating its potential impact on disease progression and patient outcomes.
Purpose: This systematic review and meta-analysis aimed to assess the impact of apabetalone in patients with cardiovascular disease.
Methods: The meta-analysis was registered with PROSPERO (CRD42023423298). Databases searched included Cochrane CENTRAL, PubMed, Ovid Medline, and Web of Science. Three unique RCTs with total of 2897 patients were included, with trial durations ranging from 12 to 124 weeks. Primary outcomes assessed were all-cause mortality and non-fatal myocardial infarction (MI). Data analysis was performed using the inverse variance common effect model. Subgroup analyses were conducted based on the presence of atherosclerotic cardiovascular disease (ASCVD) or its increased risk.
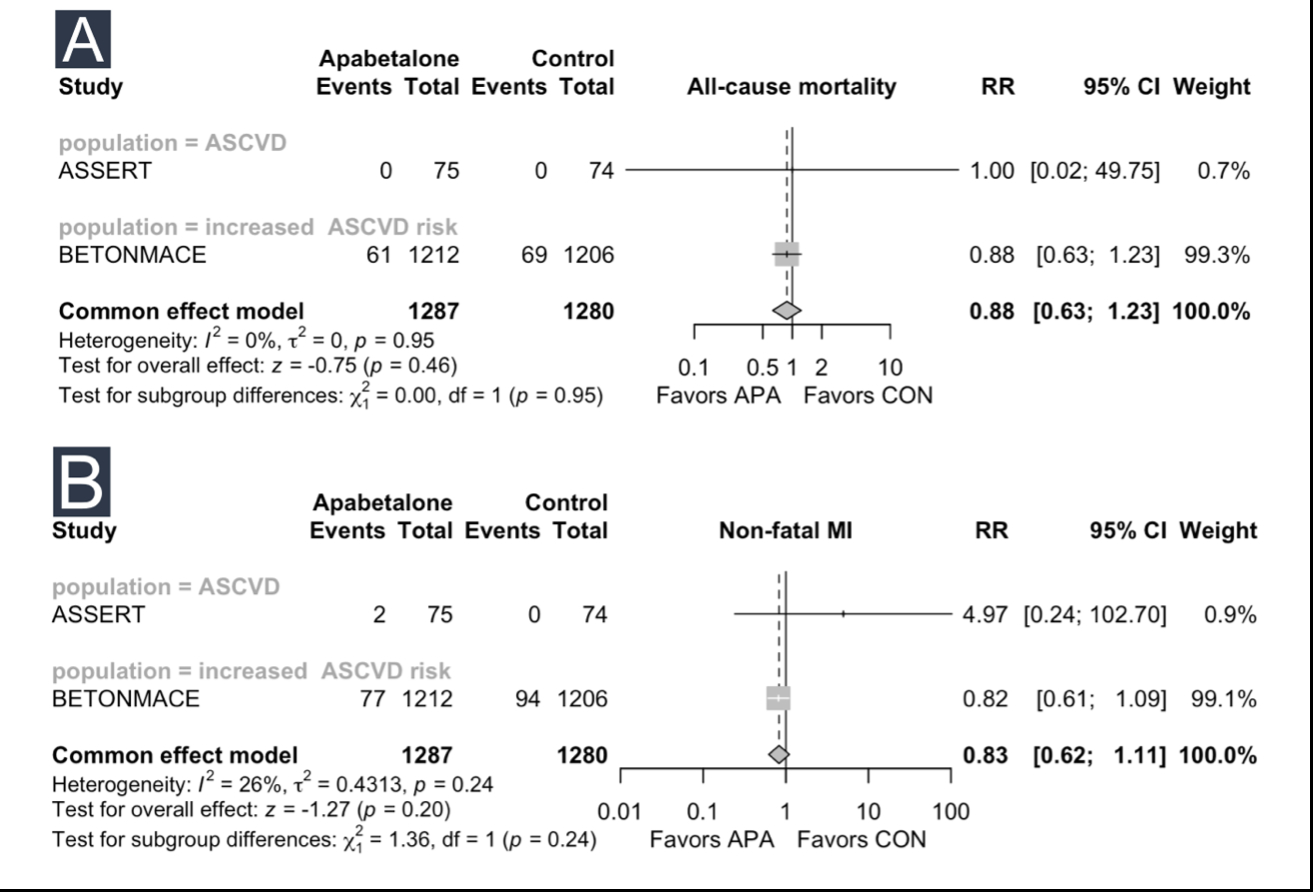
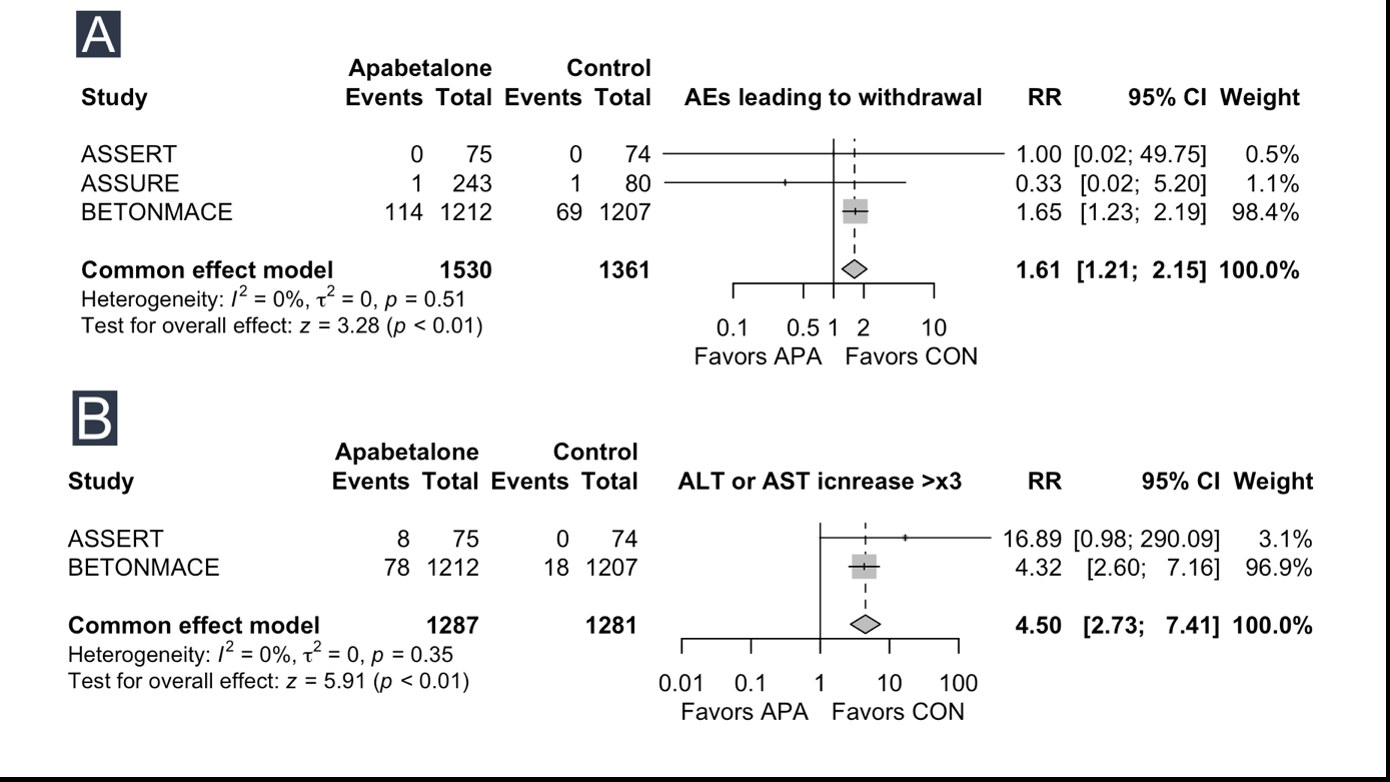
Results: Among the primary outcomes, no significant effects of apabetalone were observed on all-cause mortality (RR 0.88, 95% CI 0.63 to 1.23) or non-fatal MI (RR 0.83, 95% CI 0.62 to 1.11). Apabetalone demonstrated favorable effects on increasing Apo A1 (SMD 0.20, 95% CI 0.04 to 0.35) and HDL (SMD 0.29, 95% CI 0.09 to 0.49). However, no significant effects were observed for changes in Apo B, LDL, non-HDL, total cholesterol. Subgroup analysis did not reveal any significant differences in outcomes. Significant effects were noted in secondary outcomes. Apabetalone showed a significant increase in adverse events (AEs) leading to withdrawal (RR 1.61, 95% CI 1.21 to 2.15) and ALT and AST increases greater than three times the normal value (RR 4.50, 95% CI 2.73 to 7.41).
Conclusion: Our meta-analysis highlights the significant impact of apabetalone on certain lipid parameters and adverse events. However, it did not demonstrate significant effects on all-cause mortality or non-fatal MI. Further well-designed and reported RCTs were warranted to elucidate the clinical implications of apabetalone in selected group of patients that might benefit the most from apabetalone that may also improve cardiovascular disease management.
Purpose: This systematic review and meta-analysis aimed to assess the impact of apabetalone in patients with cardiovascular disease.
Methods: The meta-analysis was registered with PROSPERO (CRD42023423298). Databases searched included Cochrane CENTRAL, PubMed, Ovid Medline, and Web of Science. Three unique RCTs with total of 2897 patients were included, with trial durations ranging from 12 to 124 weeks. Primary outcomes assessed were all-cause mortality and non-fatal myocardial infarction (MI). Data analysis was performed using the inverse variance common effect model. Subgroup analyses were conducted based on the presence of atherosclerotic cardiovascular disease (ASCVD) or its increased risk.
Results: Among the primary outcomes, no significant effects of apabetalone were observed on all-cause mortality (RR 0.88, 95% CI 0.63 to 1.23) or non-fatal MI (RR 0.83, 95% CI 0.62 to 1.11). Apabetalone demonstrated favorable effects on increasing Apo A1 (SMD 0.20, 95% CI 0.04 to 0.35) and HDL (SMD 0.29, 95% CI 0.09 to 0.49). However, no significant effects were observed for changes in Apo B, LDL, non-HDL, total cholesterol. Subgroup analysis did not reveal any significant differences in outcomes. Significant effects were noted in secondary outcomes. Apabetalone showed a significant increase in adverse events (AEs) leading to withdrawal (RR 1.61, 95% CI 1.21 to 2.15) and ALT and AST increases greater than three times the normal value (RR 4.50, 95% CI 2.73 to 7.41).
Conclusion: Our meta-analysis highlights the significant impact of apabetalone on certain lipid parameters and adverse events. However, it did not demonstrate significant effects on all-cause mortality or non-fatal MI. Further well-designed and reported RCTs were warranted to elucidate the clinical implications of apabetalone in selected group of patients that might benefit the most from apabetalone that may also improve cardiovascular disease management.
More abstracts on this topic:
9p21.3 variants drive coronary calcification by suppressing statherin expression
Soheili Fariborz, Almontashiri Naif, Heydarikhorneh Niloufar, Vilmundarson Ragnar, Chen Hsiao-huei, Stewart Alexandre
A Genome-wide CRISPRi Screen Implicates Coronary Artery Disease GWAS Genes as Key Regulators of Adventitial Fibroblast ProliferationJackson William, Zhu Ashley, Gu Wenduo, Berezowitz Alexa, Iyer Meghana, Cheng Paul