Final ID: MDP24
LDL-C Lowering with Evolocumab and Arterial Aneurysms: Long-Term Analysis from the FOURIER Trial
Arterial aneurysms, particularly abdominal aortic aneurysms (AAA), are life-threatening conditions. Human genetic studies and preclinical mouse models have supported LDL-C reduction through PCSK9i as a strategy to slow the progression of arterial aneurysms.
AIMS
To investigate the rates of arterial aneurysm events among patients randomized to the PCSK9i evolocumab vs. placebo.
METHODS
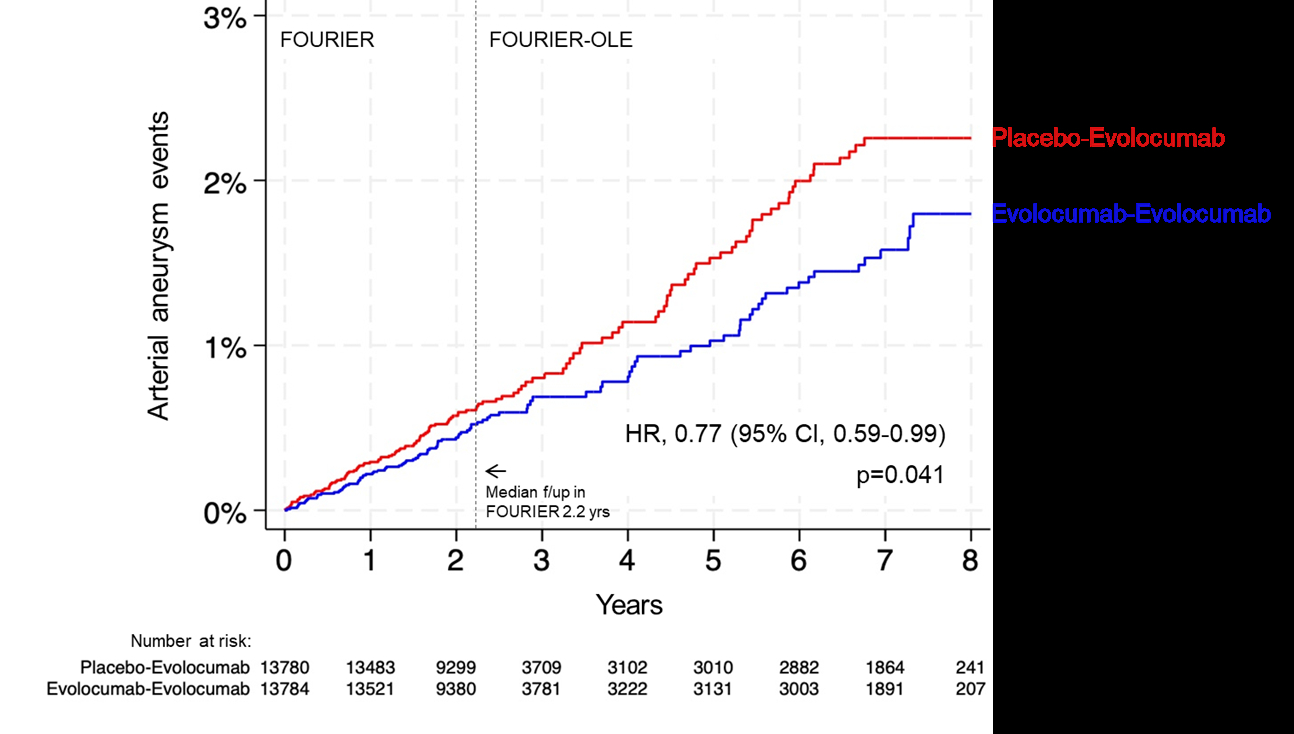
In FOURIER, 27,564 patients with stable ASCVD and LDL-C ≥70 or non-HDL-C ≥100 mg/dL on statins were randomized to evolocumab vs. placebo and followed for a median of 2.2 years. 6,635 patients entered the open-label extension period and received evolocumab for 5 years, irrespective of initial assignment. The primary endpoint of this analysis was any aneurysm event, defined as diagnosis, progression, or intervention related to an arterial aneurysm. Adverse events were reported by the local site and potential aneurysm events were centrally identified by blinded investigators. Procedure-related pseudoaneurysms were excluded. A Cox model, adjusted for randomization stratification factors and an indicator for inclusion in the open-label cohort, was used to compare randomized groups throughout the two trial periods.
RESULTS
A total of 236 patients had aneurysm events (58% AAA) during follow-up. Patients who had aneurysm events were older (median age 67 vs. 63 yrs), more likely to be male (86% vs. 75%) and have a history of PAD (21% vs. 13%). Patients randomized to evolocumab had significantly lower rates of aneurysm events during follow-up (HR 0.77 [0.59–0.99]; p=0.041) (Figure). The association between randomization to evolocumab and fewer aneurysm events was consistent for both AAA (HR 0.78 [0.56–1.09]) and non-AAA (HR 0.81 [0.55–1.18]).
CONCLUSION
In patients with stable ASCVD on optimized statin therapy, early initiation of long-term evolocumab was associated with fewer arterial aneurysm events compared with delayed initiation. These data support earlier intensive LDL-C reduction with PCSK9i as a promising strategy to prevent the formation and progression of arterial aneurysms, including AAA.
- Gaba, Prakriti ( Brigham , Boston , Massachusetts , United States )
- Keech, Anthony ( NHMRC CLINICAL TRIALS CENTRE , Camperdown Sydney , New South Wales , Australia )
- Sabatine, Marc ( TIMI Study Group, Brigham & Women's Hospital , Boston , Massachusetts , United States )
- Marston, Nicholas ( Brigham And Womens Hospital , Boston , Massachusetts , United States )
- Bergmark, Brian ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Zimerman, Andre ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- O'donoghue, Michelle ( TIMI Study Group , Boston , Massachusetts , United States )
- Giugliano, Robert ( TIMI Study Group , Boston , Massachusetts , United States )
- Murphy, Sabina ( TIMI Study Group, Brigham & Women's Hospital , Boston , Massachusetts , United States )
- Kuder, Julia ( TIMI Study Group , Boston , Massachusetts , United States )
- Monsalvo, Maria Laura ( AMGEN INC , Westlake Village , California , United States )
- Flores-arredondo, Jose ( AMGEN Inc. , Thousand Oaks , California , United States )
- Atar, Dan ( OSLO UNIVERSITY HOSPITAL ULLEVAL , Oslo , Norway )
Meeting Info:
Session Info:
Clinical and Translational Studies of Lipoproteins and Lipids in Vascular Diseases
Saturday, 11/16/2024 , 12:50PM - 02:15PM
Moderated Digital Poster Session
More abstracts on this topic:
Pirruccello James
18F-NaF and 18F-FDG and calcification predict the development of abdominal aortic aneurysms and is attenuated by drug therapyNakahara Takehiro, Miyazawa Raita, Iwabuchi Yu, Tonda Kai, Narula Nupoor, Strauss Harry, Narula Jagat, Jinzaki Masahiro
More abstracts from these authors:
Marston Nicholas, Zhang Shuanglu, Goodrich Erica, Murphy Sabina, Xia Shuting, Li Dan, Tsimikas Sotirios, Giugliano Robert, Sabatine Marc, Bergmark Brian, Alexander Vickie, Prohaska Thomas, Kang Yu Mi, Moura Filipe, Zimerman Andre, Waldman Elaine, Weinland Julia
Association Between Lipoprotein(a) Levels and Incident Complex Coronary Revascularization Procedures in the FOURIER TrialGaba Prakriti, Sabatine Marc, Bergmark Brian, O'donoghue Michelle, Giugliano Robert, Bellavia Andrea, Monsalvo Maria Laura, Flores-arredondo Jose, Kuder Julia, Atar Dan, Keech Anthony