Final ID: Su1076
Sodium-glucose cotransporter 2 inhibitors use and outcomes in transthyretin amyloid cardiomyopathy
Abstract Body (Do not enter title and authors here):
Background
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have demonstrated to reduce the risk of hospitalizations from heart failure (HF) and cardiovascular mortality. However, SGLT2i therapy’s potential effects on transthyretin amyloid cardiomyopathy (ATTR-CM) have not been well studied.
Objective
This study aims to investigate the association of SGLT2-inhibitors with outcomes in patients with transthyretin amyloid cardiomyopathy.
Methods
The TriNeTX Global Collaborative Network research database was used to identify patients aged ≥18 years of age from January 2000 to April 2023. Patients were categorized into two groups, one with transthyretin amyloid cardiomyopathy on SGLT2i and a control group with transthyretin amyloid cardiomyopathy without SGLT2i. Patients were followed for 1 month and 1 year respectively. Propensity score-matched analysis (PSM) (1:1) was performed on age, gender, race, BMI, hypertension, diabetes mellitus, chronic kidney disease, smoking status, hemoglobin level, low density lipid (LDL) level, left ventricular ejection fraction, pro-BNP levels and various drugs including ACEi, ARBi, beta-blockers, diuretics and statins. Primary outcome was all-cause mortality (ACM), while secondary outcomes were heart failure (HF), ischemic stroke, atrial fibrillation (AF) and ventricular tachycardia (VT).
Results
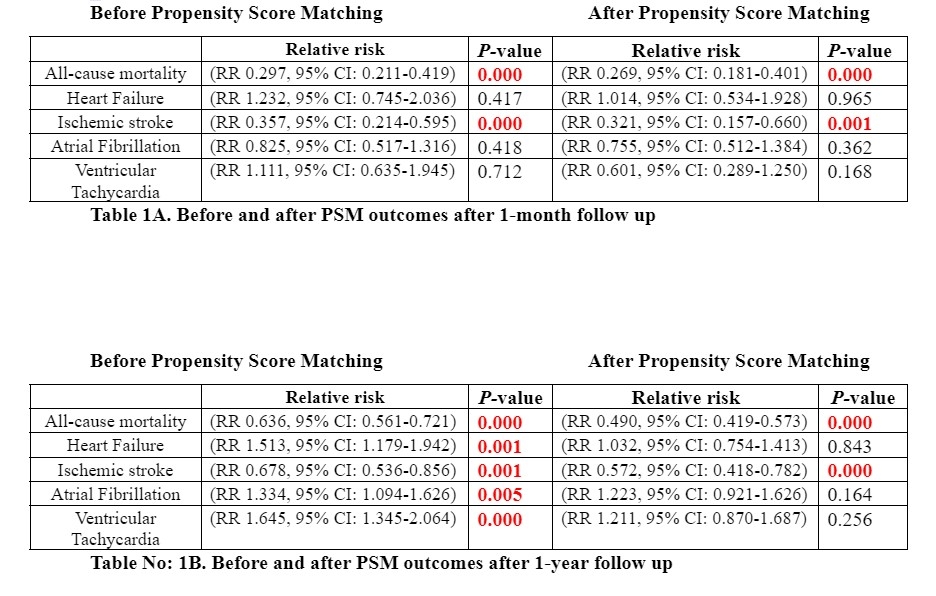
After 1:1 propensity score matching, the study cohort comprised 2,153 patients in SGLT2i and 2,153 patients in the control group. The mean age of patients in SGLT2i and control group was 74.2 and 74.4 years. PSM analysis showed that SGLT2-inhibitors in ATTR-CM patients were significantly associated with lower risk of ACM after 1 month (RR, 0.26 (95%CI: 0.18-0.40), P<0.001), and after 1 year (RR, 0.49 (95% CI: 0.41-0.57), P<0.001) compared with a control group. A similar trend was observed with a significant reduction in the risk of ischemic stroke after 1 month (RR 0.321, 95% CI: 0.157-0.660), P<0.001), and after 1 year (RR, 0.572, 95% CI: 0.418-0.782), P=0.001). However, the risk of HF, AF, and VT both at 1 month and 1 year follow-up was comparable between the SGLT2i group and control group among ATTR-CM patients.
Conclusions
These findings suggest that SGLT2i use among transthyretin amyloid cardiomyopathy patients is associated with reduced risk of mortality and ischemic stroke, but not atrial fibrillation and heart failure.
Background
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have demonstrated to reduce the risk of hospitalizations from heart failure (HF) and cardiovascular mortality. However, SGLT2i therapy’s potential effects on transthyretin amyloid cardiomyopathy (ATTR-CM) have not been well studied.
Objective
This study aims to investigate the association of SGLT2-inhibitors with outcomes in patients with transthyretin amyloid cardiomyopathy.
Methods
The TriNeTX Global Collaborative Network research database was used to identify patients aged ≥18 years of age from January 2000 to April 2023. Patients were categorized into two groups, one with transthyretin amyloid cardiomyopathy on SGLT2i and a control group with transthyretin amyloid cardiomyopathy without SGLT2i. Patients were followed for 1 month and 1 year respectively. Propensity score-matched analysis (PSM) (1:1) was performed on age, gender, race, BMI, hypertension, diabetes mellitus, chronic kidney disease, smoking status, hemoglobin level, low density lipid (LDL) level, left ventricular ejection fraction, pro-BNP levels and various drugs including ACEi, ARBi, beta-blockers, diuretics and statins. Primary outcome was all-cause mortality (ACM), while secondary outcomes were heart failure (HF), ischemic stroke, atrial fibrillation (AF) and ventricular tachycardia (VT).
Results
After 1:1 propensity score matching, the study cohort comprised 2,153 patients in SGLT2i and 2,153 patients in the control group. The mean age of patients in SGLT2i and control group was 74.2 and 74.4 years. PSM analysis showed that SGLT2-inhibitors in ATTR-CM patients were significantly associated with lower risk of ACM after 1 month (RR, 0.26 (95%CI: 0.18-0.40), P<0.001), and after 1 year (RR, 0.49 (95% CI: 0.41-0.57), P<0.001) compared with a control group. A similar trend was observed with a significant reduction in the risk of ischemic stroke after 1 month (RR 0.321, 95% CI: 0.157-0.660), P<0.001), and after 1 year (RR, 0.572, 95% CI: 0.418-0.782), P=0.001). However, the risk of HF, AF, and VT both at 1 month and 1 year follow-up was comparable between the SGLT2i group and control group among ATTR-CM patients.
Conclusions
These findings suggest that SGLT2i use among transthyretin amyloid cardiomyopathy patients is associated with reduced risk of mortality and ischemic stroke, but not atrial fibrillation and heart failure.
More abstracts on this topic:
3-Minute Heart Health App: A Feasibility Study
Abdulkarim Iya, Metzger Joseph, Stovitz Steven, Van't Hof Jeremy
A Case of Clozapine-Induced Myocarditis: An Under-described Side EffectIbrahim Rand, Clearo Kellie