Final ID: Su1116
The Supplementary Anti-Obesity Medication Integration into a Longitudinal Weight Loss (SAIL) Program: Early Experience in a Remote Comprehensive Weight Management Solution In Patients with CardioMetabolic Risk
Abstract Body (Do not enter title and authors here): Background: There is substantial imbalance between the prevalence and treatment of overweight/obesity. Team-based remote care programs have shown promise in closing healthcare delivery gaps for several cardiometabolic disorders, but whether this strategy can enhance the uptake of guideline-directed therapy for weight management remains uncertain.
Methods: In this quality improvement program, we developed and deployed a remote, patient navigator and pharmacist-led, pharmacotherapy-oriented weight management intervention (Supplementary Anti-Obesity Integration into a Longitudinal Weight Loss [SAIL] program). SAIL was conducted within the Partnerships for Reducing Overweight and Obesity with Patient-Centered Strategies 2.0 (PROPS 2.0) program, an ongoing 12-month digital health program pairing an online weight management program (RestoreHealth; HealthFleet, Inc.) with personalized support from health coaches. After 6 months, PROPS 2.0 participants who did not experience weight reduction were offered possible enrollment in SAIL. Pharmacists, enabled by a collaborative drug therapy management program, prescribed, titrated, and monitored anti-obesity medications (AOM) with physician (cardiologist) supervision.
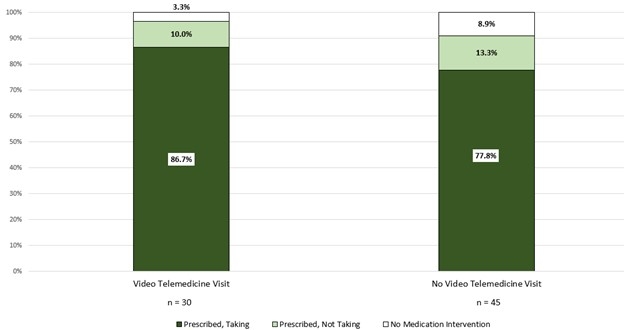
Results: Overall, 2,540 invitations for participation in SAIL were sent to the 5,061 patients enrolled in PROPS 2.0, of whom 200 responded. Of the respondents, 98 (49%) were eligible for SAIL, and 75 patients were enrolled. Based randomly by enrollment period, 45 patients participated without a remote physician visit, while 30 had a video telemedicine visit. Among the 75 program participants, 70 (93%) received a prescription for AOM (29/30 with a visit vs. 41/45 without; P=0.64). After a median follow-up of 143 days (IQR 79-193), 61/70 were taking prescribed AOM (26/29 with a visit vs. 35/41 without; P=0.73) (Figure).
Conclusion: This study extends prior experiences leveraging remote, team-based care, emphasizing the potential of this approach to enhance weight management. Given the dramatic cardiometabolic detriments of prolonged exposure to overweight and obesity, innovative approaches are necessary to meet demand. Remote and team-based care are proven methods to improve care and outcomes and may provide a novel model for delivering care for overweight and obesity. Further studies are needed to ascertain the effectiveness of this strategy on weight-related health outcomes.
Methods: In this quality improvement program, we developed and deployed a remote, patient navigator and pharmacist-led, pharmacotherapy-oriented weight management intervention (Supplementary Anti-Obesity Integration into a Longitudinal Weight Loss [SAIL] program). SAIL was conducted within the Partnerships for Reducing Overweight and Obesity with Patient-Centered Strategies 2.0 (PROPS 2.0) program, an ongoing 12-month digital health program pairing an online weight management program (RestoreHealth; HealthFleet, Inc.) with personalized support from health coaches. After 6 months, PROPS 2.0 participants who did not experience weight reduction were offered possible enrollment in SAIL. Pharmacists, enabled by a collaborative drug therapy management program, prescribed, titrated, and monitored anti-obesity medications (AOM) with physician (cardiologist) supervision.
Results: Overall, 2,540 invitations for participation in SAIL were sent to the 5,061 patients enrolled in PROPS 2.0, of whom 200 responded. Of the respondents, 98 (49%) were eligible for SAIL, and 75 patients were enrolled. Based randomly by enrollment period, 45 patients participated without a remote physician visit, while 30 had a video telemedicine visit. Among the 75 program participants, 70 (93%) received a prescription for AOM (29/30 with a visit vs. 41/45 without; P=0.64). After a median follow-up of 143 days (IQR 79-193), 61/70 were taking prescribed AOM (26/29 with a visit vs. 35/41 without; P=0.73) (Figure).
Conclusion: This study extends prior experiences leveraging remote, team-based care, emphasizing the potential of this approach to enhance weight management. Given the dramatic cardiometabolic detriments of prolonged exposure to overweight and obesity, innovative approaches are necessary to meet demand. Remote and team-based care are proven methods to improve care and outcomes and may provide a novel model for delivering care for overweight and obesity. Further studies are needed to ascertain the effectiveness of this strategy on weight-related health outcomes.
More abstracts on this topic:
Albuminuria Drives Type 2 Diabetes-Related Atrial Fibrillation: an ACCORD substudy
Siqueira Amanda, Everett Brendan
A Community Outreach Program Focused on Hypertension Awareness Reaches 600+ People in Rural Georgia and Works to Build the Next Generation of Biomedical ScientistsDent Elena, Ilatovskaya Daria, Pinkerton Brittany, Crider Emily, Ryan Michael, Sullivan Jennifer