Final ID: Mo1103
Are We Including Everybody? A Systematic Review On Reporting Of Transgender and Gender-Expansive Populations in Cardiovascular Clinical Trials
Abstract Body (Do not enter title and authors here): BACKGROUND: Transgender and gender expansive (TGE) people constitute a vulnerable population with added cardiovascular risk and frequently experience health inequalities and obstacles to care. However, they are nonetheless underreported and underrepresented in clinical trials. We aim to understand inclusion and representation of TGE population in clinical research by examining the volume and caliber of reporting in certain disciplines of CV clinical trials.
METHODS: A systematic review of clinical trials from January 2020 to March 2024 was conducted across 4 cardiovascular areas (heart failure, stroke, dyslipidemia, electrophysiology) using ClinicalTrials.gov focusing on the inclusion and reporting of TGE populations in the United States with published results. Trials were evaluated for explicit mention of TGE participants, use of gender-inclusive language, and provision of disaggregated data by gender identity. Equity metrics, such as the proportion of trials including TGE participants were calculated.
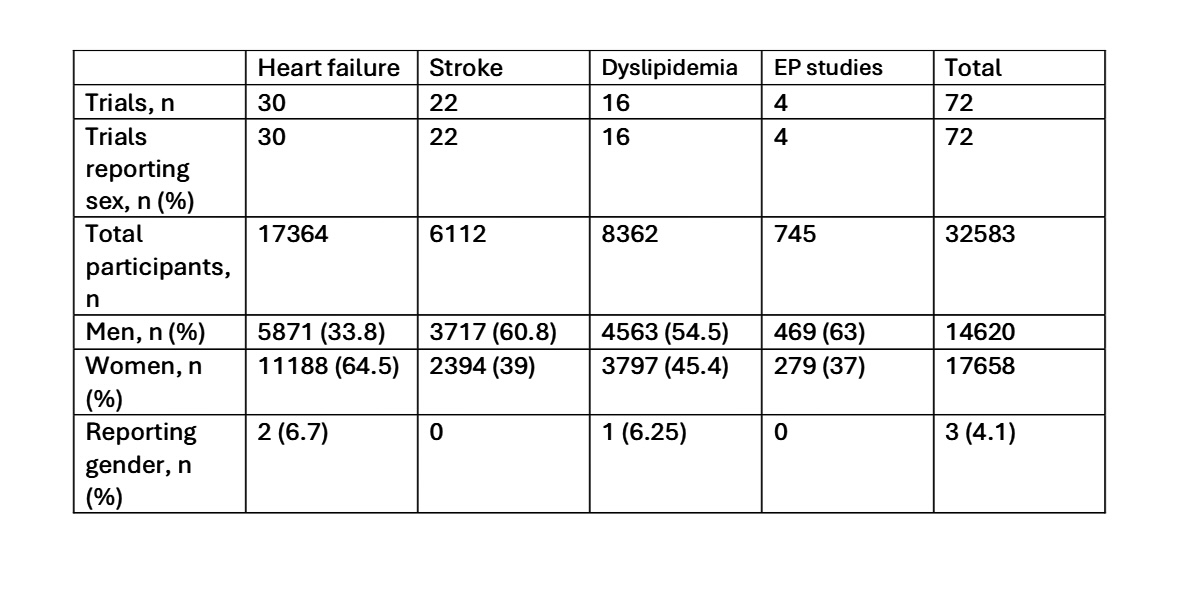
RESULTS: A total of 32,583 participants were included(Table).We collected reporting of populations for sex and gender descriptions, as well as the inclusion and exclusion criteria. Almost all studies reported sex or gender as a single outcome variable. We inferred this to denote sex unless it was specified otherwise. All studies reported sex, with women representing 54% of all participants. Women represented the minority of study participants in stroke (39%), dyslipidemia (45%), and electrophysiology (37%) clinical trials, but majority of participants in heart failure trials (64%). Gender identity, including the option “other”, was reported in only 3 trials (4.1%), of which 2 were heart failure and 1 was a dyslipidemia trial. Most trials had pregnant women, lactating women, and age <18 years as exclusion criteria, and had adults aged >18 years as the inclusion criteria.
CONCLUSION: Our findings confirm that there is still a lack of commitment in current clinical trials to the Sex and Gender Equity in Research guidelines. The impact of the differences in this population can never be fully understood unless gender identification data is carefully gathered. Current national guidelines mandate that in randomized clinical trials, gender should be fully reported and that gender-inclusive language be used appropriately. To properly represent and care for these populations, future cardiology studies should adhere to the Sex and Gender Equity in Research principles.
METHODS: A systematic review of clinical trials from January 2020 to March 2024 was conducted across 4 cardiovascular areas (heart failure, stroke, dyslipidemia, electrophysiology) using ClinicalTrials.gov focusing on the inclusion and reporting of TGE populations in the United States with published results. Trials were evaluated for explicit mention of TGE participants, use of gender-inclusive language, and provision of disaggregated data by gender identity. Equity metrics, such as the proportion of trials including TGE participants were calculated.
RESULTS: A total of 32,583 participants were included(Table).We collected reporting of populations for sex and gender descriptions, as well as the inclusion and exclusion criteria. Almost all studies reported sex or gender as a single outcome variable. We inferred this to denote sex unless it was specified otherwise. All studies reported sex, with women representing 54% of all participants. Women represented the minority of study participants in stroke (39%), dyslipidemia (45%), and electrophysiology (37%) clinical trials, but majority of participants in heart failure trials (64%). Gender identity, including the option “other”, was reported in only 3 trials (4.1%), of which 2 were heart failure and 1 was a dyslipidemia trial. Most trials had pregnant women, lactating women, and age <18 years as exclusion criteria, and had adults aged >18 years as the inclusion criteria.
CONCLUSION: Our findings confirm that there is still a lack of commitment in current clinical trials to the Sex and Gender Equity in Research guidelines. The impact of the differences in this population can never be fully understood unless gender identification data is carefully gathered. Current national guidelines mandate that in randomized clinical trials, gender should be fully reported and that gender-inclusive language be used appropriately. To properly represent and care for these populations, future cardiology studies should adhere to the Sex and Gender Equity in Research principles.
More abstracts on this topic:
A ChatGLM-based stroke diagnosis and prediction tool
Song Xiaowei, Wang Jiayi, Ma Weizhi, Wu Jian, Wang Yueming, Gao Ceshu, Wei Chenming, Pi Jingtao
6-Nitrodopamine potentiates the positive chronotopic and inotropic effect induced by noradrenaline in the rat isolated heartLima Antonio, Sobanski Joao Fernando, Antunes Edson, De Nucci Gilberto