Final ID: Su2072
Use of Cardiac Magnetic Resonance Imaging to Assess Response to Oral Treprostinil Therapy in Pediatric Pulmonary Hypertension Patients1
Abstract Body (Do not enter title and authors here): Background:
Pulmonary hypertension (PH) contributes to significant morbidity and mortality in pediatric patients. Cardiac catheterization remains standard of care for diagnosis and serial monitoring in PH patients despite its significant risk of morbidity and mortality. Cardiac magnetic resonance (CMR) imaging provides critical information about the right ventricle (RV) and left ventricle (LV) and may have significant prognostic power. We aim to use CMR to demonstrate response to oral Treprostinil in pediatric PH patients.
Methods:
This is a retrospective, multi-center study of 9 PH patients who underwent a baseline CMR study, as well as a follow up study 24 weeks following transitioning to oral Treprostinil from intravenous/inhaled formulations. Feature-tracking (Qstrain, Medis) was performed on short/long-axis cines to assess RV global longitudinal strain (GLS), LV GLS, and LV global circumferentialstrain (GCS). Volumetric data and conventional functional parameters were also compared, pre and post initiation of oral Treprostinil therapy.
Results:
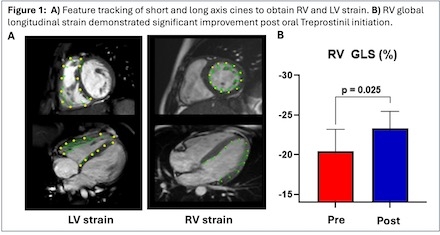
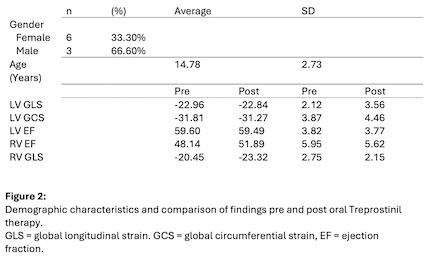
Eighteen CMR studies from 9 patients were analyzed. Average baseline RV ejection fraction (EF) was 48.1% compared to post therapy RV EF 51.9% (p = 0.188). Average baseline RV GLS was -20.5% compared to post therapy RV GLS -23.3% (p = 0.025), Figure 1. Overall, there was no significant difference in LV EF, LV GLS, and LV GCS after transitioning to oral therapy. Increased RV EF post initiation of oral Treprostinil therapy correlated with increased magnitude LV GCS (r = 0.701, p = 0.036), reflecting critical interventricular interaction in the disease process.
Conclusion:
Assessment of RV strain is more sensitive in detecting improvement in cardiac performance following initiation of oral Treprostinil in pediatric PH patients compared to ejection fraction. Future studies should focus on the development of novel CMR biomarkers in place of cardiac catheterization in assessing cardiac performance in PH patients.
1. This abstract was written by one of ISS investigators, Dr. Truong, who is using data from TDE-PH-206 (pediatric oral Treprostinil study) to investigate cMRI parameters.
Pulmonary hypertension (PH) contributes to significant morbidity and mortality in pediatric patients. Cardiac catheterization remains standard of care for diagnosis and serial monitoring in PH patients despite its significant risk of morbidity and mortality. Cardiac magnetic resonance (CMR) imaging provides critical information about the right ventricle (RV) and left ventricle (LV) and may have significant prognostic power. We aim to use CMR to demonstrate response to oral Treprostinil in pediatric PH patients.
Methods:
This is a retrospective, multi-center study of 9 PH patients who underwent a baseline CMR study, as well as a follow up study 24 weeks following transitioning to oral Treprostinil from intravenous/inhaled formulations. Feature-tracking (Qstrain, Medis) was performed on short/long-axis cines to assess RV global longitudinal strain (GLS), LV GLS, and LV global circumferentialstrain (GCS). Volumetric data and conventional functional parameters were also compared, pre and post initiation of oral Treprostinil therapy.
Results:
Eighteen CMR studies from 9 patients were analyzed. Average baseline RV ejection fraction (EF) was 48.1% compared to post therapy RV EF 51.9% (p = 0.188). Average baseline RV GLS was -20.5% compared to post therapy RV GLS -23.3% (p = 0.025), Figure 1. Overall, there was no significant difference in LV EF, LV GLS, and LV GCS after transitioning to oral therapy. Increased RV EF post initiation of oral Treprostinil therapy correlated with increased magnitude LV GCS (r = 0.701, p = 0.036), reflecting critical interventricular interaction in the disease process.
Conclusion:
Assessment of RV strain is more sensitive in detecting improvement in cardiac performance following initiation of oral Treprostinil in pediatric PH patients compared to ejection fraction. Future studies should focus on the development of novel CMR biomarkers in place of cardiac catheterization in assessing cardiac performance in PH patients.
1. This abstract was written by one of ISS investigators, Dr. Truong, who is using data from TDE-PH-206 (pediatric oral Treprostinil study) to investigate cMRI parameters.
More abstracts on this topic:
A Novel EMR-Based Algorithm with the Virtual Echocardiography Screening Tool (VEST) to Screen Patients for Pulmonary Arterial Hypertension
Narowska Gabriela, Anand Suneesh, Gangireddy Chethan, Enevoldsen John, Keane Martin, Edmundowicz Daniel, Forfia Paul, Vaidya Anjali
2 Dimensional Echocardiography versus 3 Dimentional Echocardiography to Assess Right Ventricular Function in Pulmonary Hypertension: A Systematic ReviewChaudhry Waleed Razzaq, Hajj Fatima, Bathula Satyamedha, Meghji Mohammed Askari, Pasupuleti Hemalatha, Kiyani Madiha, Shah Syeda Simrah, Neelakantan Ramaswamy Sanathanan, Mirzaeidizaji Nakisa, St. Jacques Jahnoy, Khan Khalil Ullah, Veluchamy Elakkiya, Jesse Joshanna