Final ID: 4144573
Cardiac biomarkers, intensive lifestyle intervention, and risk of heart failure subtypes in type 2 diabetes – a post-hoc analysis of the Look AHEAD trial
Methods: Adults with T2D and overweight/obesity in the Look Action for Health in Diabetes (AHEAD) trial without prevalent HF were included. NT-proBNP and hs-cTnT were measured at baseline, 1- and 4-years (Roche Diagnostics). Adjusted Cox models were created to evaluate the associations of baseline, 1- and 4-year change in NT-proBNP and hs-cTnT with risk of HF with preserved and reduced ejection fraction (HFpEF and HFrEF, respectively). Interaction testing was performed to evaluate heterogeneous effects of the ILI vs diabetes support and education (DSE) across baseline cardiac biomarkers.
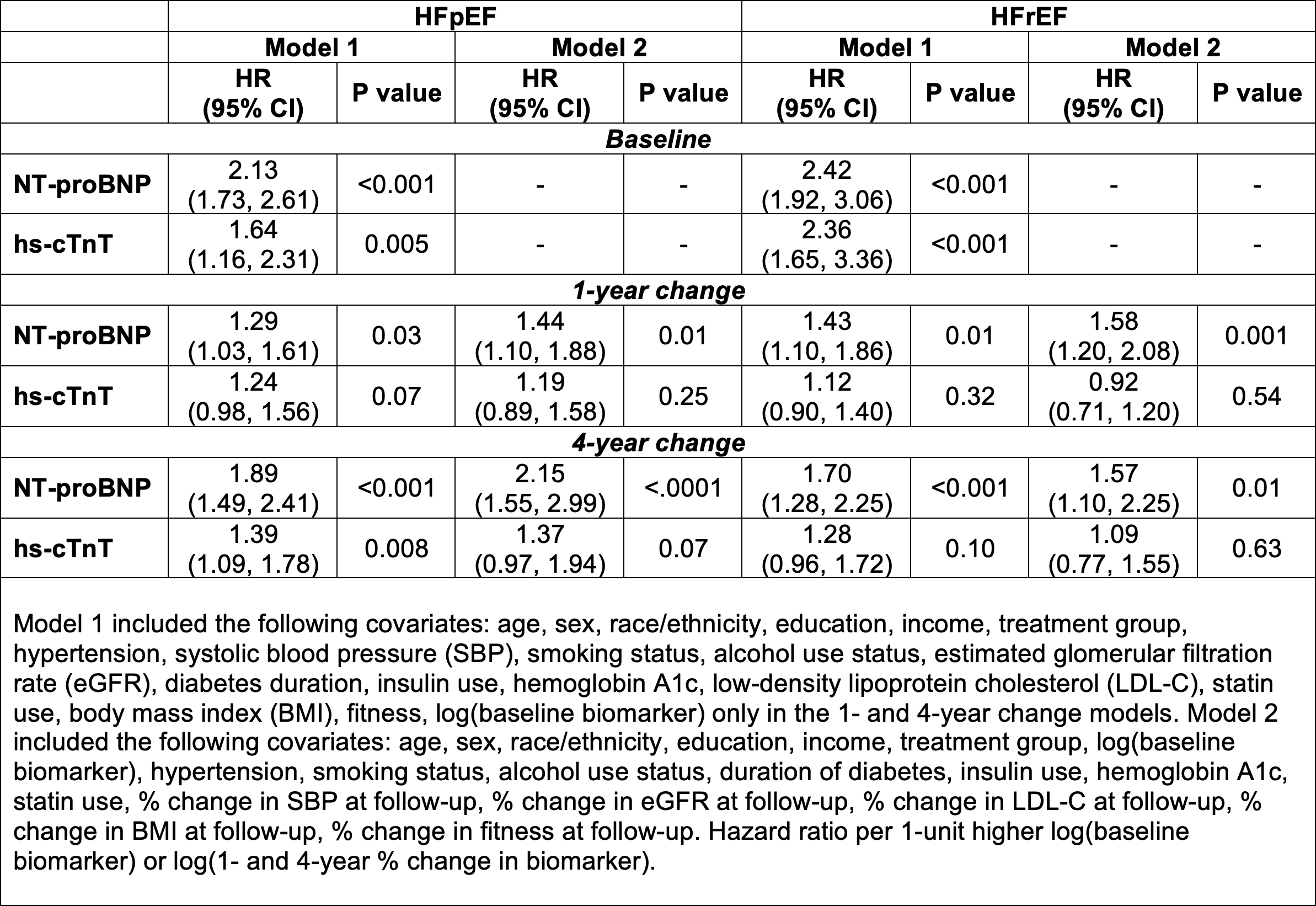
Results: Of the 3,959 participants included, 212 had incident HF (108 HFpEF, 84 HFrEF) over 12 years. Higher baseline NT-proBNP and hs-cTnT were each significantly associated with higher risk of HFpEF and HFrEF (Table). Increases in NT-proBNP over 1- and 4-years were significantly associated with higher risk of HFpEF and HFrEF with a similar pattern of association for hs-cTnT and HF subtypes. After accounting for risk factor changes, the association of 1- and 4-year changes in NT-proBNP, but not hs-cTnT, with risk of HF subtypes remained significant. There was a significant interaction between NT-proBNP and ILI for risk of HFpEF but not HFrEF (p-int = 0.001). The ILI reduced HFpEF risk among participants with elevated (≥125 pg/mL) but not non-elevated NT-proBNP (<125 pg/mL) (ILI vs DSE HR [95% CI]: 0.48 [0.25-0.93] and 1.67 [0.94-2.94], respectively). No significant treatment group by hs-cTnT interactions were identified.
Conclusions: In T2D, longitudinal changes in cardiac biomarkers, particularly NT-proBNP, are significantly associated with HF risk, independent of risk factor changes. In Look AHEAD, elevated NT-proBNP may identify individuals who are more likely to benefit from the ILI for HFpEF prevention.
- Chunawala, Zainali ( UT SOUTHWESTERN MEDICAL CENTER , Dallas , Texas , United States )
- Bertoni, Alain ( Wake Forest Univ Med Schl , Winston Salem , North Carolina , United States )
- Espeland, Mark ( Wake Forest Univ Med Schl , Winston Salem , North Carolina , United States )
- Pandey, Ambarish ( UT SOUTHWESTERN MEDICAL CENTER , Dallas , Texas , United States )
- Patel, Kershaw ( Houston Methodist , Houston , Texas , United States )
- Segar, Matthew ( Texas Heart Institute , Houston , Texas , United States )
- Garcia, Katelyn ( Wake Forest Univ Med Schl , Winston Salem , North Carolina , United States )
- Bhatt, Deepak ( Mount sinai Fuster Heart Hospital , Newyork city , New York , United States )
- Wang, Thomas ( UT SOUTHWESTERN MEDICAL CENTER , Dallas , Texas , United States )
- Januzzi, James ( Massachusetts General Hospital , Wellesley Hills , Massachusetts , United States )
- Butler, Javed ( Baylor Scott and White Research Institute , Dallas , Texas , United States )
- Lam, Carolyn ( NATIONAL HEART CENTRE SINGAPORE , Singapore , Singapore )
Meeting Info:
Session Info:
Saturday, 11/16/2024 , 09:45AM - 11:00AM
Abstract Oral Session
More abstracts on this topic:
Li Zhen, Doiron Jake, Xia Huijing, Lapenna Kyle, Sharp Thomas, Yu Xiaoman, Nagahara Noriyuki, Goodchild Traci, Lefer David
A multi-proteomic Risk Score Predicts Adverse Cardiovascular Outcomes in Patients with Angina and Non-obstructive Coronary Artery DiseaseHuang Jingwen, Lodhi Rafia, Lodhi Saleha, Eldaidamouni Ahmed, Hritani Wesam, Hasan Muhammet, Haroun Nisreen, Quyyumi Arshed, Mehta Puja, Leon Ana, Ko Yi-an, Yang Huiying, Medina-inojosa Jose, Ahmed Taha, Harris Kristen, Alkhoder Ayman, Al Kasem Mahmoud
More abstracts from these authors:
Patel Kershaw, Bertoni Alain, Espeland Mark, Pandey Ambarish, Chunawala Zainali, Segar Matthew, Garcia Katelyn, Ndumele Chiadi, Wang Thomas, Januzzi James, Butler Javed, Lam Carolyn
Sex Differences in the Association of Baseline and Longitudinal Changes in Cardiac Biomarkers with Risk of Heart Failure subtypes – a post-hoc analysis of the Look AHEAD trialChunawala Zainali, Garcia Katelyn, Wang Thomas, Bayes-genis Antoni, Pandey Ambarish, Patel Lajjaben, Keshvani Neil, Segar Matthew, Espeland Mark, Ballantyne Christie, Januzzi James, Lam Carolyn, Bertoni Alain