Final ID: MDP121
Effect of the Cardiac Myosin Activator Omecamtiv Mecarbil on Risk of Ventricular Arrhythmias in Heart Failure with Reduced Ejection Fraction: the GALACTIC-HF Trial
The selective cardiac myosin activator, omecamtiv mecarbil (OM), has been shown to benefit individuals with HFrEF but the clinical experience of cardiac myosin activators and incidence of ventricular arrhythmias (VA) is limited. We investigated the prognostic role of VA and the associations between OM, VA occurrence, and outcomes in the GALACTIC-HF trial.
Methods:
GALACTIC-HF was a placebo-controlled trial testing the efficacy and safety of OM in participants with symptomatic chronic HF and LVEF≤35%. VA were captured based on safety adverse event reporting. Severe HF was defined according to the ESC-HFA criteria. Poisson regression models using restricted cubic splines model were used to assess the association between baseline LVEF and incidence rates of VA. Associations between incident VA and subsequent clinical outcomes were evaluated using time-updated Cox proportional hazard models.
Results:
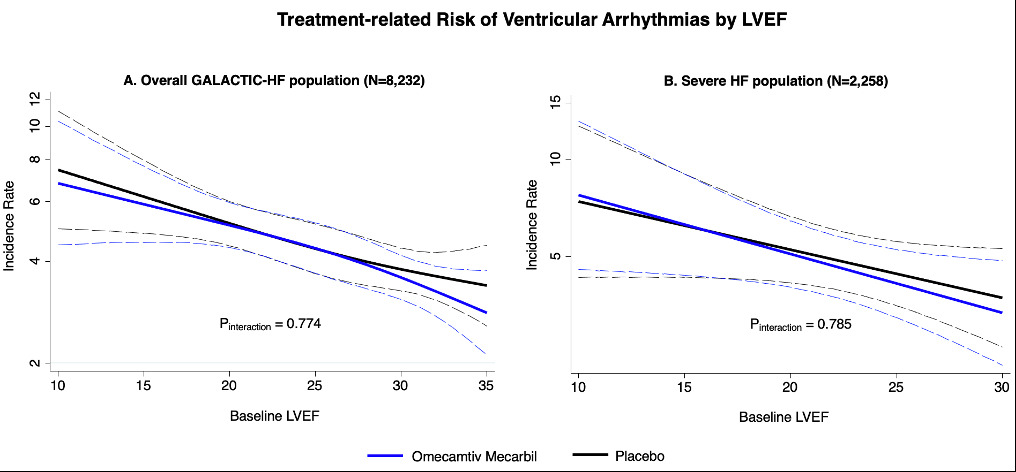
Over a median follow-up of 21.8 months, 594 out of the 8,232 individuals randomized in the GALACTIC-HF trial experienced VA, with no statistically significant differences between study arms. Individuals with lower LVEF values were at greater risk of incident VA (per 10% decline: HR 1.37; 95% CI 1.21-1.55; p<0.001) with no significant differences between study arms (Figure, panel A). Following VA occurrence, participants were at higher risk of the primary endpoint of CV death or HF events (adj HR 1.67; 95% CI 1.42-1.97; p<0.001) and all-cause mortality (adj HR 2.07; 95% CI 1.77-2.42; p<0.001). The treatment effects of OM on clinical outcomes were consistent in patients not experiencing VA and after VA events. Among the 2,258 participants with severe HF, OM did not affect the risk of VA (adj HR 0.95; 95% CI 0.70-1.29) and this was consistent across the range of LVEF; Figure, panel B.
Conclusions:
The occurrence of VA among patients with HFrEF is associated with a substantially higher risk of subsequent fatal and non-fatal events. OM did not increase the risk of VA and the beneficial treatment effects were maintained irrespective of VA occurrence. These findings reinforce the safety of OM use among individuals with HFrEF who are at risk of arrhythmic events.
- Foa', Alberto ( Brigham and Women's Hospital, Harvard Medical School, Boston MA , Boston , Massachusetts , United States )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Teerlink, John ( SAN FRANCISCO VAMC UCSF , San Francisco , California , United States )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Mani, Govini ( Cytokinetics Inc. , Portland , Oregon , United States )
- Diaz, Rafael ( Estudios Clínicos Latino América (ECLA) , Rosario , Argentina )
- Malik, Fady ( CYTOKINETICS INC. , S San Fran , California , United States )
- Heitner, Stephen ( Cytokinetics Inc. , Portland , Oregon , United States )
- Felker, Gary ( DUKE CLINICAL RESEARCH INSTITUTE , Durham , North Carolina , United States )
- Metra, Marco ( UNIVERSITY OF BRESCIA , Brescia , Italy )
Meeting Info:
Session Info:
Bulking Up: The Latest in Hypertrophic Cardiomyopathy
Saturday, 11/16/2024 , 02:50PM - 04:05PM
Moderated Digital Poster Session
More abstracts on this topic:
Taleb Shayandokht, Hsu Jamie, Saver Jeffrey
A Rare Cause of Recurrent Heart Failure Exacerbations After Transcatheter Aortic Valve Replacement: Ventricular Septal Defect and Significant Paravalvular LeakMedina Jesse, Vincent Louis, Rodriguez Ferreira Esteban, Spence-miller Shanice, Fernandez Joel, Colombo Rosario, Calfa Marian
More abstracts from these authors:
Lu Henri, Vaduganathan Muthiah, Solomon Scott, Claggett Brian, Felker Gary, Mcmurray John, Teerlink John, Metra Marco, Heitner Stephen, Diaz Rafael, Malik Fady
Lactate dehydrogenase and clinical outcomes in patients with heart failure and reduced ejection fraction: Insights from the GALACTIC-HF trialOno Ryohei, Malik Fady, Felker Gary, Henderson Alasdair David, Jhund Pardeep, Vaduganathan Muthiah, Solomon Scott, Teerlink John, Mcmurray John, Yang Mingming, Docherty Kieran, Chimura Misato, Metra Marco, Liu Genzhou, Divanji Punag, Heitner Stephen, Kupfer Stuart