Final ID: Mo2156
Efficacy and Safety of Omecamtiv Mecarbil in Heart Failure with Reduced Ejection Fraction According to Age: the GALACTIC-HF Trial
Purpose: To assess the CV outcomes, treatment response and tolerability to OM according to age, in patients enrolled in the GALACTIC-HF trial.
Methods: GALACTIC-HF was a randomized, double-blind clinical trial testing OM vs placebo in patients with symptomatic HF. Key eligibility criteria included an age between 18 and 85 years, elevated natriuretic peptides and a left ventricular ejection fraction (LVEF) ≤35%. We examined the treatment effect of OM vs placebo for the primary endpoint of CV death or first HF event (hospitalization or urgent visit for HF), in the overall population and severe HF subgroup (LVEF≤30%, New York Heart Association [NYHA] Class III/IV, HF hospitalization within 6 months), as well as safety outcomes according to predefined age groups (<65 or ≥65).
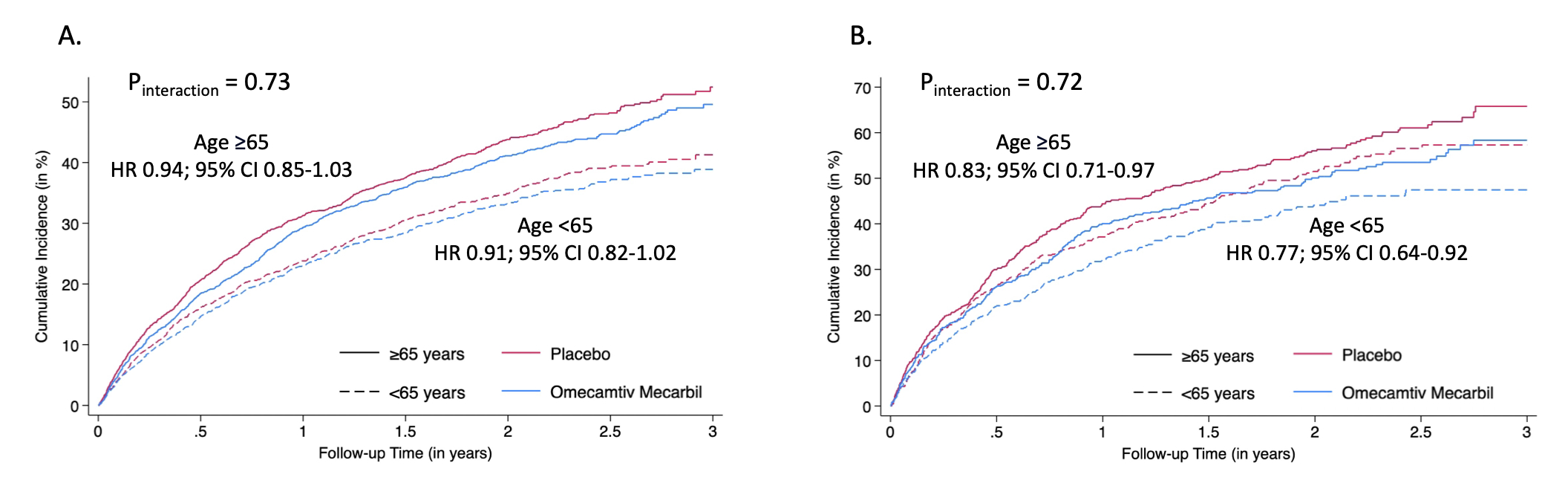
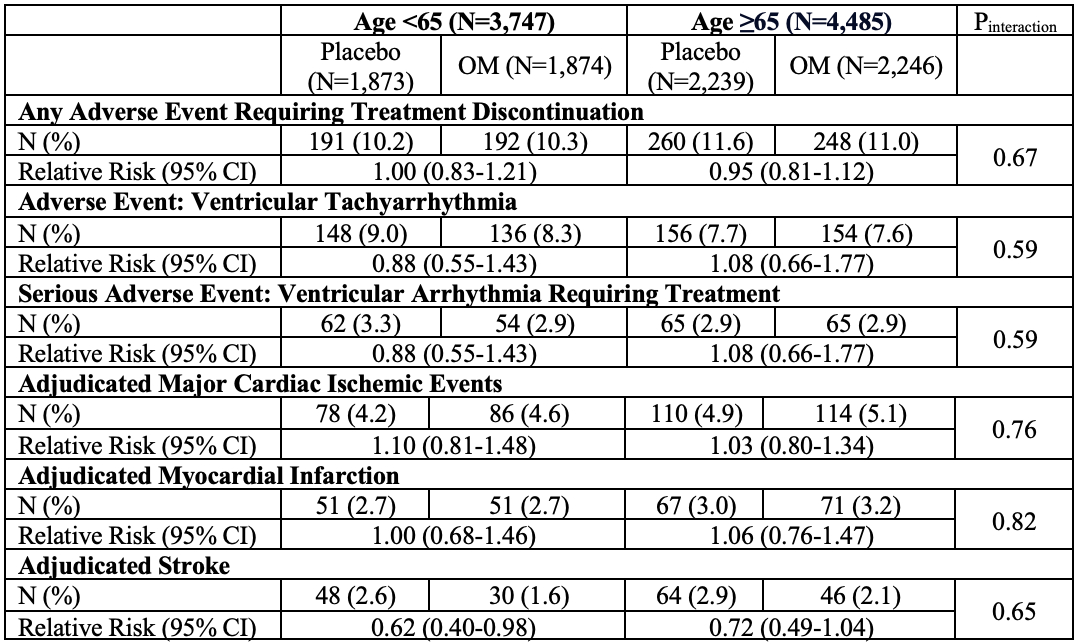
Results: A total of 8,232 patients (age 64.5±11.4 years, 54.5% ≥65 years, 21% women) were included. The rate of the primary outcome was 21.4 per 100py in age <65 years and 28.8 per 100py in age ≥65 years. The treatment effect of OM for the primary outcome (hazard ratio [HR] 0.92; 95% confidence interval [CI] 0.86-0.99; p=0.025) was consistent in age groups (Pinteraction=0.73, Figure 1 Panel A) and irrespective of age as a continuous variable (Pinteraction=0.19), after adjustment for treatment interactions with known modifiers of the effects of OM (LVEF and baseline atrial fibrillation status). In patients with severe HF, OM significantly reduced the risk of the primary outcome in both age <65 years (HR 0.77; 95% CI 0.64-0.92) and age ≥65 years (HR 0.83; 95% CI 0.71-0.97; Pinteraction=0.72, Figure 1 Panel B). The safety profile of OM was consistent irrespective of age (Pinteraction>0.05 for all, Table 1).
Conclusions: In GALACTIC-HF, older individuals were well-represented and faced higher risks of CV events. Treatment with OM was safe irrespective of age, and reduced the risk of CV death or first HF event across the age spectrum, especially in those with severe HF.
- Lu, Henri ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Felker, Gary ( DUKE CLINICAL RESEARCH INSTITUTE , Durham , North Carolina , United States )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Teerlink, John ( SAN FRANCISCO VAMC UCSF , San Francisco , California , United States )
- Metra, Marco ( UNIVERSITY OF BRESCIA , Brescia , Italy )
- Heitner, Stephen ( Cytokinetics Inc. , Portland , Oregon , United States )
- Diaz, Rafael ( Estudios Clinicos Latino América, Instituto Cardiovascular de Rosario , Rosario , Argentina )
- Malik, Fady ( Cytokinetics Inc. , Portland , Oregon , United States )
Meeting Info:
Session Info:
Taking Action to Understand the Ejection Fraction
Monday, 11/18/2024 , 10:30AM - 11:30AM
Abstract Poster Session
More abstracts on this topic:
Pergola Pablo, Szarek Michael, Zayed Hany, Hemani Famina, Degoma Emil, Andorfer Cathy, Walsh John, Ridker Paul, Bhatt Deepak
A Phase 2a randomized controlled trial of once-daily versus twice-daily remote ischemic conditioning in vascular cognitive impairment (TRIC-VCI)Ganesh Aravind, Mccreary Cheryl, Sahlas Demetrios, Sharma Mukul, Swartz Richard, Smith Eric, Barber Philip, Black Sandra, Corbett Dale, Field Thalia, Frayne Richard, Hachinski Vladimir, Ismail Zahinoor, Mai Lauren
More abstracts from these authors:
Foa' Alberto, Mcmurray John, Teerlink John, Solomon Scott, Vaduganathan Muthiah, Claggett Brian, Mani Govini, Diaz Rafael, Malik Fady, Heitner Stephen, Felker Gary, Metra Marco
Lactate dehydrogenase and clinical outcomes in patients with heart failure and reduced ejection fraction: Insights from the GALACTIC-HF trialOno Ryohei, Malik Fady, Felker Gary, Henderson Alasdair David, Jhund Pardeep, Vaduganathan Muthiah, Solomon Scott, Teerlink John, Mcmurray John, Yang Mingming, Docherty Kieran, Chimura Misato, Metra Marco, Liu Genzhou, Divanji Punag, Heitner Stephen, Kupfer Stuart