Final ID: Mo1120
Hispanic/Latino Patients with Heart Failure with Preserved Ejection Fraction: A Participant Level Pooled Analysis of 3 Contemporary Trials
Prior studies have suggested that Hispanic/Latino patients with HFpEF in the US may have lower mortality rates than non-Hispanic/Latinos. However, few contemporary data exist examining the clinical risks faced by Hispanic/Latino patients with HFpEF, especially in global settings.
Methods:
We performed a participant-level pooled analysis of the TOPCAT (Americas region), PARAGON-HF, and DELIVER trials, including participants with symptomatic HF and an LVEF >40% (in DELIVER) or ≥45% (in TOPCAT and PARAGON-HF). Hispanic/Latino ethnicity was self-reported. The risk of the primary endpoint, cardiovascular death or HF hospitalization, was compared among Hispanic/Latino and non-Hispanic/Latino patients using Cox regression models adjusted for relevant clinical covariates, stratified by trial and region of enrollment.
Results:
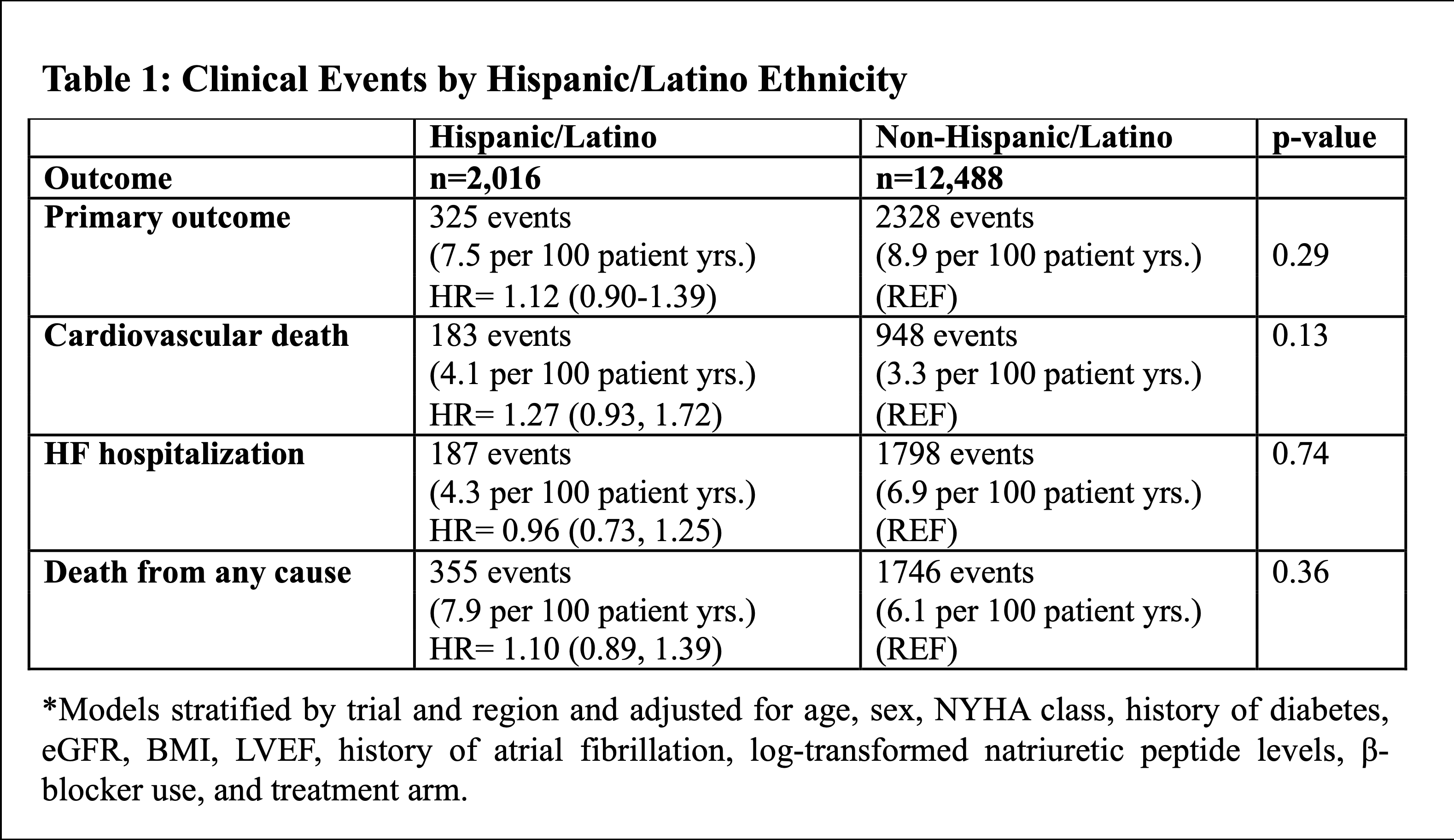
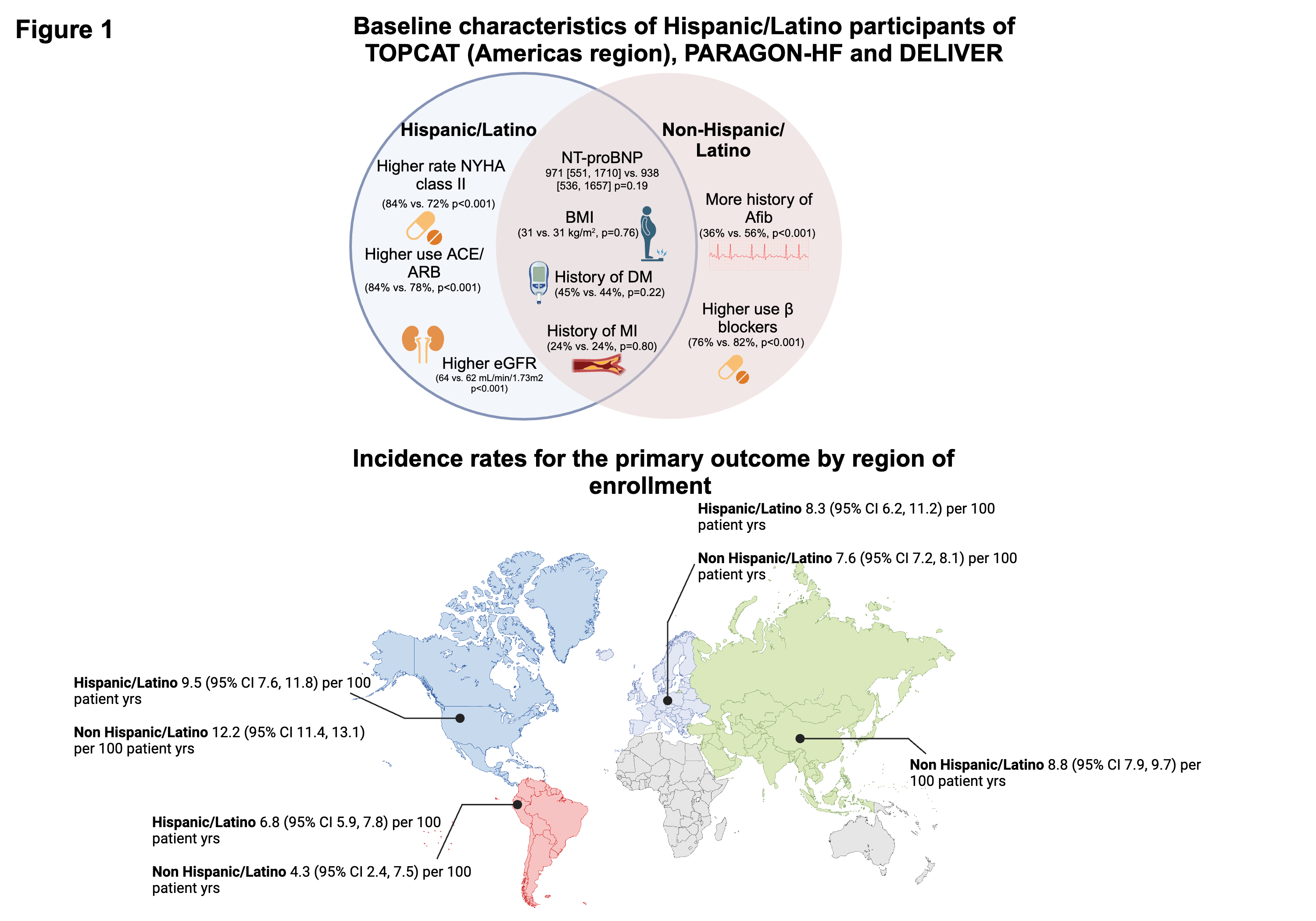
Of the 12,826 participants, 2,013 (16%) were Hispanic/Latino. 71% of Hispanic/Latino patients were enrolled in Latin America, 19% in North America and 10% in Europe. Hispanic/Latino participants were less likely to have a history of atrial fibrillation (36% vs. 56%), had a higher eGFR, and had better functional status but had similar levels of natriuretic peptides as compared to non-Hispanic patients (Figure 1). During a median follow-up of 2.6 years, Hispanic/Latino participants experienced similar rates of the primary outcome (adj HR 1.12; 95% CI 0.90-1.39; p=0.29) and secondary outcomes as compared to non-Hispanic/Latinos (Table 1 and Figure 1). Findings were similar when including only patients enrolled in the Americas (n=4,728; adj HR 1.05; 95% CI: 0.78-1.41; p=0.77). There were no interactions with age, sex, BMI, or diabetes status.
Conclusions:
Contrary to prior reports, this pooled analysis of HFpEF participants enrolled in 3 contemporary global trials showed that Hispanic/Latino patients experienced similarly heightened risks of adverse cardiovascular outcomes and mortality as non-Hispanic/Latino patients.
- Pabon, Maria ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Mcmurray, John ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Fernandez, Marcos ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Makuvire, Tracy ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Lewis, Eldrin ( STANFORD UNIVERSITY , Palo Alto , California , United States )
- Jhund, Pardeep ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Pfeffer, Marc ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Pitt, Bertram ( University of Michigan School , Ann Arbor , Michigan , United States )
Meeting Info:
Session Info:
HFPEF Potpourri: Latest Advances and Emerging Topics
Monday, 11/18/2024 , 10:30AM - 11:30AM
Abstract Poster Session
More abstracts on this topic:
Ganesh Aravind, Mccreary Cheryl, Sahlas Demetrios, Sharma Mukul, Swartz Richard, Smith Eric, Barber Philip, Black Sandra, Corbett Dale, Field Thalia, Frayne Richard, Hachinski Vladimir, Ismail Zahinoor, Mai Lauren
Black and Hispanic Patients Have Lower Survival Outcomes Than White Patients in Out-of-Hospital Cardiac Arrests Witnessed by 9-1-1 RespondersPande Madhura, Ornato Joseph, Powell Stephen, Starks Monique, Yow Eric, Chan Paul, Blewer Audrey, Mark Daniel, Al-khalidi Hussein, Sasson Comilla, Mcnally Bryan, Al-araji Rabab
More abstracts from these authors:
Ostrominski John, Pitt Bertram, Zannad Faiez, Zile Michael, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Lassen Mats, Butt Jawad, Claggett Brian, Anand Inder, Desai Akshay, Jhund Pardeep, Lam Carolyn, Pfeffer Marc
Age, Adiposity-Related Anthropometrics, and Clinical Outcomes in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: A Participant-Level Pooled Analysis of Randomized TrialsOstrominski John, Pitt Bertram, Zannad Faiez, Zile Michael, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Lassen Mats, Butt Jawad, Claggett Brian, Anand Inder, Desai Akshay, Jhund Pardeep, Lam Carolyn, Pfeffer Marc