Final ID: Su3005
Age, Adiposity-Related Anthropometrics, and Clinical Outcomes in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: A Participant-Level Pooled Analysis of Randomized Trials
Methods: In this participant-level pooled analysis of five international, multicenter, randomized-controlled trials enrolling adults with HF and either mildly reduced or preserved ejection fraction (HFmrEF/HFpEF), the association between age and adiposity-related anthropometrics (BMI, waist circumference [WC], and waist-to-height ratio [WHtR]) and the primary composite outcome of time-to-first HF hospitalization or cardiovascular (CV) death was evaluated using multivariable adjusted Cox proportional hazards models (stratified by treatment and trial) and restricted cubic splines.
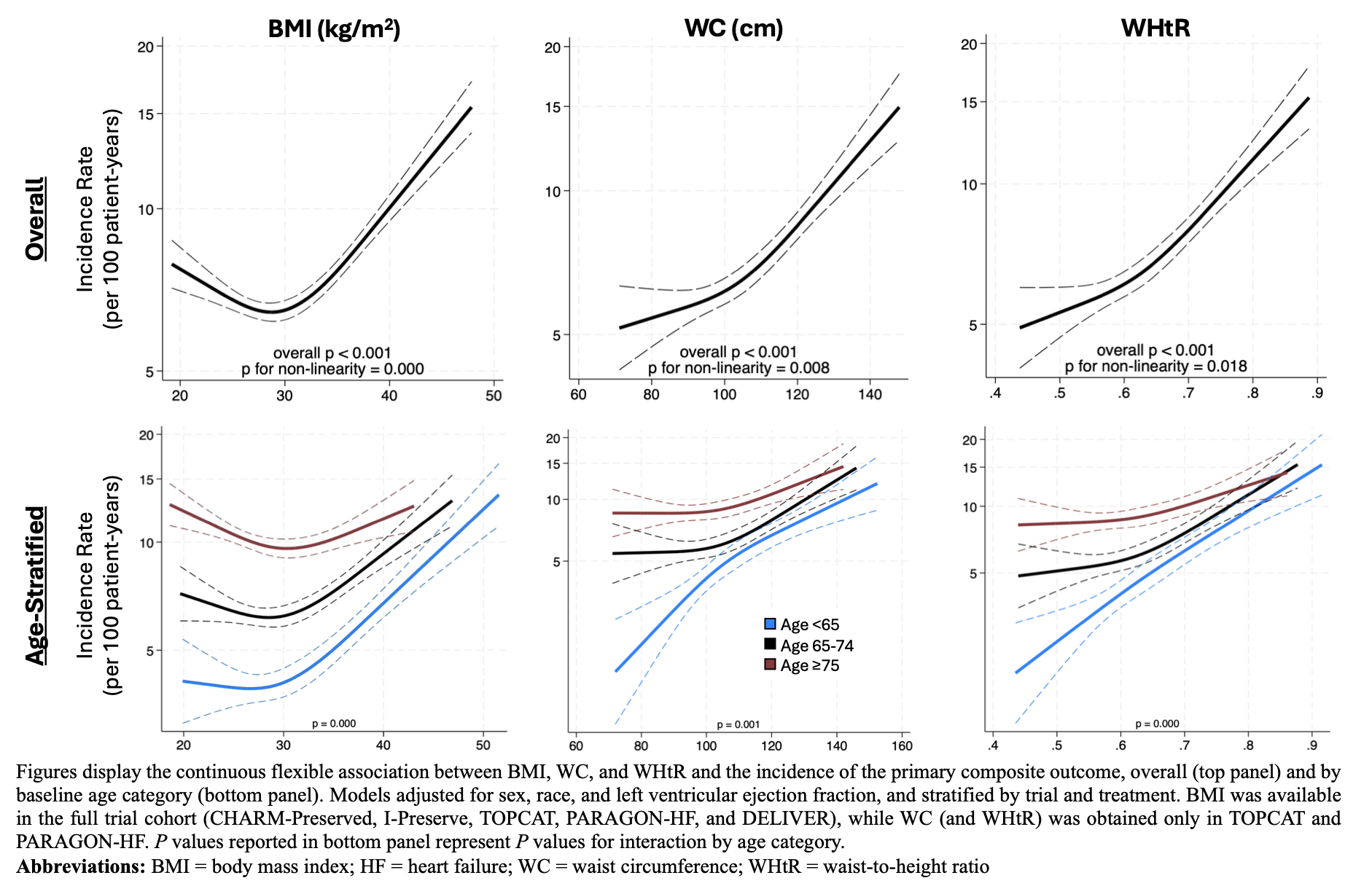
Results: Among 21,606 participants in the analysis (mean age, 71±9 years; 49% female; 83% White; mean BMI, 30±6 kg/m2), 5,350 (25%) were aged <65 years, 8,273 (38%) were aged 65-74 years, and 7,983 (37%) were aged ≥75 years at baseline. Older individuals were more likely to be female, have lower BMI and WC, and higher left ventricular ejection fraction. Overall, BMI, WC, and WHtR were steeply and non-linearly associated with the primary outcome (Figure). Age, as either a categorical or continuous variable, significantly modified the covariate-adjusted association between all anthropometrics and the primary composite outcome (P≤0.001 for all) (Figure). A J-shaped relationship, characterized by higher incidence of the primary outcome with lower anthropometric values, was observed only for BMI and was substantially attenuated among younger adults. Irrespective of the anthropometric used, increasing adiposity was most steeply associated with primary events among adults aged <65 years.
Conclusions: In this pooled analysis of HFmrEF/HFpEF trials, age was a powerful modifier of the relationship between excess adiposity and HF hospitalization or CV death. An “obesity-survival paradox” – observed only for BMI – was not apparent in younger participants, who exhibited the steepest increase in the rate of clinical events with higher adiposity.
- Ostrominski, John ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Pitt, Bertram ( University of Michigan School , Ann Arbor , Michigan , United States )
- Zannad, Faiez ( CVCT and Universite de Lorraine , Paris , France )
- Zile, Michael ( MEDICAL UNIV OF SOUTH CAROLINA , Charleston , South Carolina , United States )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Lassen, Mats ( GENTOFTE UNIVERSITY HOSPITAL , Hellerup , Denmark )
- Butt, Jawad ( Rigshospitalet , Copenhagen , Denmark )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Anand, Inder ( VA SAN DIEGO HEATHCARE SYSTEM , La Jolla , California , United States )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Jhund, Pardeep ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Lam, Carolyn ( NATIONAL HEART CENTRE SINGAPORE , Singapore , Singapore )
- Pfeffer, Marc ( BRIGHAM and WOMENS HOSPITAL , Boston , Massachusetts , United States )
Meeting Info:
Session Info:
Deep Dive into the Relationship between Obesity and CVD
Sunday, 11/17/2024 , 11:30AM - 12:30PM
Abstract Poster Session
More abstracts on this topic:
Li Zhen, Borch Jensen Martin, Vondriska Thomas, Lefer David, Gehred Natalie, Gromova Tatiana, Lapenna Kyle, Sharp Thomas, Chen Jingshu, Shambhu Smitha, Yu Xiaoman, Goodchild Traci
3-Mercaptopyruvate Sulfurtransferase is a Critical Regulator of Branched-Chain Amino Acid Catabolism in Cardiometabolic HFpEFLi Zhen, Doiron Jake, Xia Huijing, Lapenna Kyle, Sharp Thomas, Yu Xiaoman, Nagahara Noriyuki, Goodchild Traci, Lefer David
More abstracts from these authors:
Ostrominski John, Pitt Bertram, Zannad Faiez, Zile Michael, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Lassen Mats, Butt Jawad, Claggett Brian, Anand Inder, Desai Akshay, Jhund Pardeep, Lam Carolyn, Pfeffer Marc
Interplay Between Heart Failure Progression, New-Onset Diabetes, and Finerenone: A FINEARTS-HF AnalysisOstrominski John, Zannad Faiez, Pitt Bertram, Brinker Meike, Schloemer Patrick, Rohwedder Katja, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Lu Henri, Claggett Brian, Desai Akshay, Jhund Pardeep, Lam Carolyn, Senni Michele, Shah Sanjiv, Voors Adriaan