Final ID: MDP884
AI-enabled multimodal risk stratification of probable transthyretin amyloid cardiomyopathy from point-of-care electrocardiography and ultrasound: A report from the TRACE-AI Study
Abstract Body (Do not enter title and authors here): Introduction: Artificial intelligence (AI) tools may enable the detection of transthyretin amyloid cardiomyopathy (ATTR-CM) from 12-lead electrocardiographic (ECG) images and point-of-care ultrasonography (POCUS). The TRACE-AI study is designed to examine the burden and outcomes of potentially undiagnosed cases through the opportunistic deployment of multimodality AI algorithms.
Research Question: To explore the burden and prognosis of probable ATTR-CM identified by opportunistically deployed AI-ECG and AI-POCUS in the emergency department (ED).
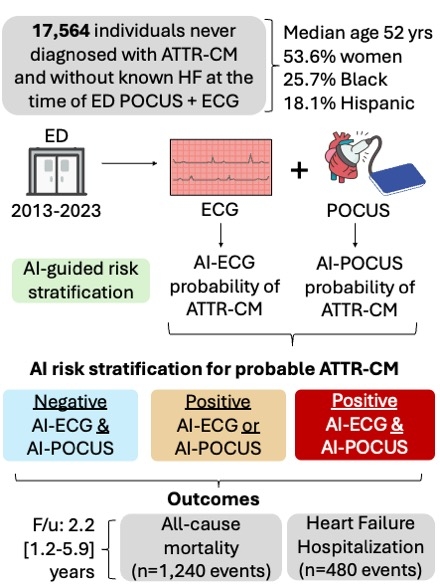
Methods: Across a diverse, 5-hospital health system, we identified individuals never diagnosed with ATTR-CM who had previously sought care in the ED during 2013-2023 and had no history of heart failure (HF) at baseline. Among these, 17,564 individuals (median age 52 [IQR: 36-66] years; 9,407 [53.6%] women, 4,520 [25.7%] Black and 3,180 [18.1%] Hispanic) had undergone a 12-lead ECG and POCUS within one day of each other (Fig. 1). We deployed validated AI algorithms for ATTR-CM from 12-lead ECG images and POCUS videos to obtain study-level probabilities of ATTR-CM. We stratified the population based on validated AI-ECG and AI-POCUS thresholds and examined the associations of screen positivity with mortality and HF hospitalization in Cox models adjusted for age, sex, hypertension, and diabetes mellitus.
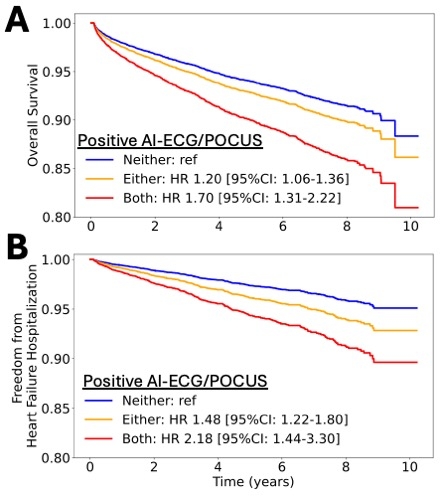
Results: Among 17,564 eligible individuals, 780 (4.4%) screened positive by AI-ECG, and 286 (1.6%) by both modalities. Over a median follow-up of 2.2 [IQR: 1.2-5.9] years, there were 1,226 deaths and 458 HF hospitalizations. Screen positivity on both modalities conferred a 1.7-fold higher adjusted risk of death (adj. HR 1.70 [95%CI: 1.31-2.22], Fig. 2A) and 2.2-fold higher risk of HF hospitalization (adj. HR 2.18 [95%CI: 1.44-3.30], Fig. 2B), compared with those screening negative on both modalities. Even among those screening positive on one modality, there was a significant 1.2-fold and 1.5-fold higher risk of death and HF hospitalization relative to the reference group (Fig 2A-B).
Conclusions: Scalable AI technology applied to ECG images and POCUS as part of their ED care identified that ~1.6% of individuals screened positive for ATTR-CM on both modalities but without eventual clinical diagnosis. This group of patients with probable undiagnosed ATTR-CM had a higher risk of long-term adverse outcomes. This underscores the need for improving ATTR-CM diagnostic rates through scalable technology applied in routine care.
Research Question: To explore the burden and prognosis of probable ATTR-CM identified by opportunistically deployed AI-ECG and AI-POCUS in the emergency department (ED).
Methods: Across a diverse, 5-hospital health system, we identified individuals never diagnosed with ATTR-CM who had previously sought care in the ED during 2013-2023 and had no history of heart failure (HF) at baseline. Among these, 17,564 individuals (median age 52 [IQR: 36-66] years; 9,407 [53.6%] women, 4,520 [25.7%] Black and 3,180 [18.1%] Hispanic) had undergone a 12-lead ECG and POCUS within one day of each other (Fig. 1). We deployed validated AI algorithms for ATTR-CM from 12-lead ECG images and POCUS videos to obtain study-level probabilities of ATTR-CM. We stratified the population based on validated AI-ECG and AI-POCUS thresholds and examined the associations of screen positivity with mortality and HF hospitalization in Cox models adjusted for age, sex, hypertension, and diabetes mellitus.
Results: Among 17,564 eligible individuals, 780 (4.4%) screened positive by AI-ECG, and 286 (1.6%) by both modalities. Over a median follow-up of 2.2 [IQR: 1.2-5.9] years, there were 1,226 deaths and 458 HF hospitalizations. Screen positivity on both modalities conferred a 1.7-fold higher adjusted risk of death (adj. HR 1.70 [95%CI: 1.31-2.22], Fig. 2A) and 2.2-fold higher risk of HF hospitalization (adj. HR 2.18 [95%CI: 1.44-3.30], Fig. 2B), compared with those screening negative on both modalities. Even among those screening positive on one modality, there was a significant 1.2-fold and 1.5-fold higher risk of death and HF hospitalization relative to the reference group (Fig 2A-B).
Conclusions: Scalable AI technology applied to ECG images and POCUS as part of their ED care identified that ~1.6% of individuals screened positive for ATTR-CM on both modalities but without eventual clinical diagnosis. This group of patients with probable undiagnosed ATTR-CM had a higher risk of long-term adverse outcomes. This underscores the need for improving ATTR-CM diagnostic rates through scalable technology applied in routine care.
More abstracts on this topic:
A machine learning approach to examining the associations of minority stressors and physical activity among sexual and gender minority adults
Lopez Veneros David, Ensari Ipek, Bhilegaonkar Riya, Sharma Yashika, Caceres Billy
A Predictive Tool and Diagnostic Screening Algorithm for the Identification of Transthyretin Amyloid Cardiomyopathy in High-Risk Patient PopulationsChai Jocelyn, Sathananthan Janarthanan, Fine Nowell, Davis Margot, Starovoytov Andrew, Campbell Christine, Hawkins Nathaniel, Virani Sean, Luong Michael, Straatman Lynn, Kiess Marla, Worsley Daniel