Final ID: 4143980
Bleeding with the FXI Inhibitor Abelacimab compared with Rivaroxaban in Patients on Antiplatelet therapy: A Prespecified Analysis of the AZALEA-TIMI 71 Trial
Combining antiplatelet (APT) with anticoagulant therapy increases the risk of bleeding. In AZALEA-TIMI 71, the novel factor XI inhibitor abelacimab reduced the risk of bleeding compared with rivaroxaban in patients with atrial fibrillation (AF). In this analysis, we investigated whether the safety of abelacimab vs rivaroxaban was modified by antiplatelet therapy.
Methods:
AZALEA-TIMI 71 randomized 1,287 patients with AF to abelacimab (90 or 150 mg subcutaneously monthly) or rivaroxaban (20 mg orally daily), with stratification by planned use of concomitant APT. The primary outcome, major or clinically relevant non-major (CRNM) bleeding, was compared using Cox proportional hazards adjusted for age, sex, and BMI, with an interaction term for randomized treatment and APT use.
Results:
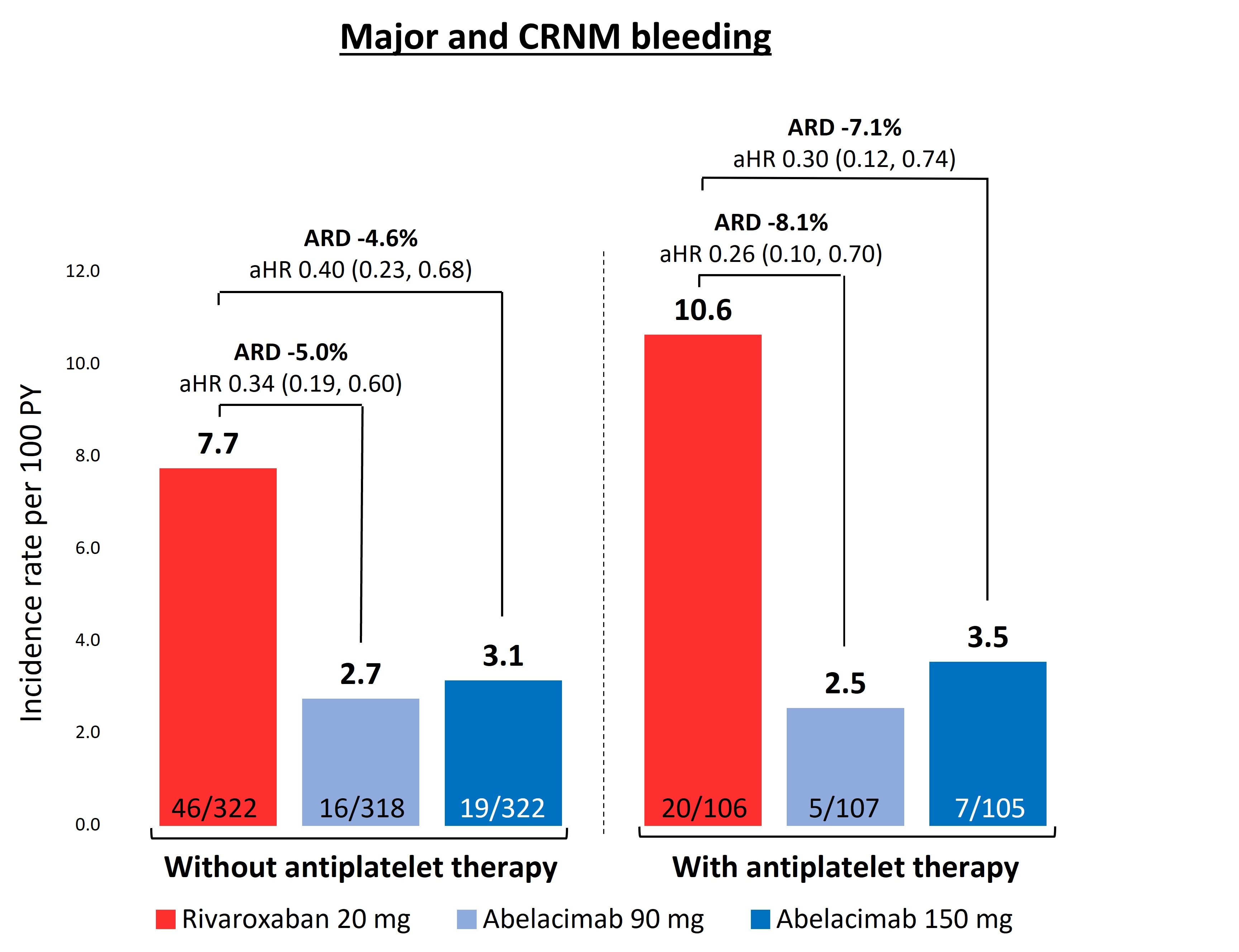
Of 1,287 patients, 318 (25%) were on APT at baseline (16% aspirin only, 8% P2Y12 only, 2% DAPT) and were younger (median age 72 vs 75 years) and had a higher prevalence of CAD (74% vs 40%), prior MI (36% vs 16%) and PAD (15% vs 11%) than those not on APT (p<0.05 for each). The rate of major or CRNM bleeding tended to be higher in those on APT than those not taking APT in the rivaroxaban group (10.6% vs. 7.7%), but not in the abelacimab group (Fig). Both abelacimab doses significantly reduced major or CRNM bleeding compared with rivaroxaban regardless of APT use [60-66% in patients not on ATP and 70-74% in patients on ATP] (Fig). Given the higher rates of bleeding in patients on both rivaroxaban and APT, the corresponding absolute risk reductions with abelacimab tended to be greater in patients on APT (7.1-8.1%) compared to those not on APT (4.6-5.0%) (Fig).
Conclusion:
Inhibition of FXI with abelacimab results in substantial reductions in bleeding compared with rivaroxaban regardless of concomitant APT use. These data support the potential advantage of FXI inhibitors in patients who require concomitant APT.
- Al Said, Samer ( TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School , Boston , Massachusetts , United States )
- Goodman, Shaun ( Division of Cardiology, Department of Medicine, St. Michael's Hospital Canadian Heart Research Centre, University of Toronto , Toronto , Ontario , Canada )
- Joung, Boyoung ( Division of Cardiology, Severance Hospital, Yonsei University College of Medicine , Seoul , Korea (the Republic of) )
- Kiss, Robert ( Department of Cardiology, Central Hospital of Northern Pest-Military Hospital, Heart and Vacular Center, Semmelweis University , Budapest , Hungary )
- Spinar, Jindrich ( University Hospital Brno , Brno , Czechia )
- Wojakowski, Wojciech ( Division of Cardiology and Structural Heart Diseases, Medical University of Silesia , Katowice , Poland )
- Weitz, Jeffrey ( Thrombosis and Atherosclerosis Research Institute. McMaster University , Hamilton , Ontario , Canada )
- Bloomfield, Dan ( Anthos Therapeutics, Inc , Cambridge , Massachusetts , United States )
- Sabatine, Marc ( TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School , Boston , Massachusetts , United States )
- Ruff, Christian ( TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School , Boston , Massachusetts , United States )
- Patel, Siddharth ( TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School , Boston , Massachusetts , United States )
- Giugliano, Robert ( TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School , Boston , Massachusetts , United States )
- Morrow, David ( TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School , Boston , Massachusetts , United States )
- Goodrich, Erica ( TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School , Boston , Massachusetts , United States )
- Murphy, Sabina ( TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School , Boston , Massachusetts , United States )
- Hug, Bruce ( Anthos Therapeutics, Inc , Cambridge , Massachusetts , United States )
- Parker, Sanobar ( Anthos Therapeutics, Inc , Cambridge , Massachusetts , United States )
- Chen, Shih-ann ( Heart Rhythm Center, Taipei Veterans General Hospital and Cardiovascular Center, Taichung Veterans Hospital; National Yang Ming Chiao Tung University and National Chung Hsing University , Taichung , Taiwan )
Meeting Info:
Session Info:
Translational Discovery in Thrombosis and Anti-thrombotic Therapeutics
Saturday, 11/16/2024 , 03:15PM - 04:30PM
Abstract Oral Session
More abstracts on this topic:
Au Michael, Zhou Mengnan, An Jaejin
A Novel Variant in GNB2 as a Cause of Sick Sinus SyndromeBulut Aybike, Karacan Mehmet, Saygili E. Alper, Pirli Dogukan, Aydin Eylul, Ozdemir Ozkan, Balci Nermin, Alanay Yasemin, Bilguvar Kaya, Akgun Dogan Ozlem
More abstracts from these authors:
Gaba Prakriti, Keech Anthony, Sabatine Marc, Marston Nicholas, Bergmark Brian, Zimerman Andre, O'donoghue Michelle, Giugliano Robert, Murphy Sabina, Kuder Julia, Monsalvo Maria Laura, Flores-arredondo Jose, Atar Dan
Olezarsen in Patients with Severe Hypertriglyceridemia: The CORE-TIMI 72a and CORE2-TIMI 72b TrialsMarston Nicholas, Zhang Shuanglu, Goodrich Erica, Murphy Sabina, Xia Shuting, Li Dan, Tsimikas Sotirios, Giugliano Robert, Sabatine Marc, Bergmark Brian, Alexander Vickie, Prohaska Thomas, Kang Yu Mi, Moura Filipe, Zimerman Andre, Waldman Elaine, Weinland Julia