Final ID: MDP913
High-Intensity Statins in Veterans with Peripheral Artery Disease
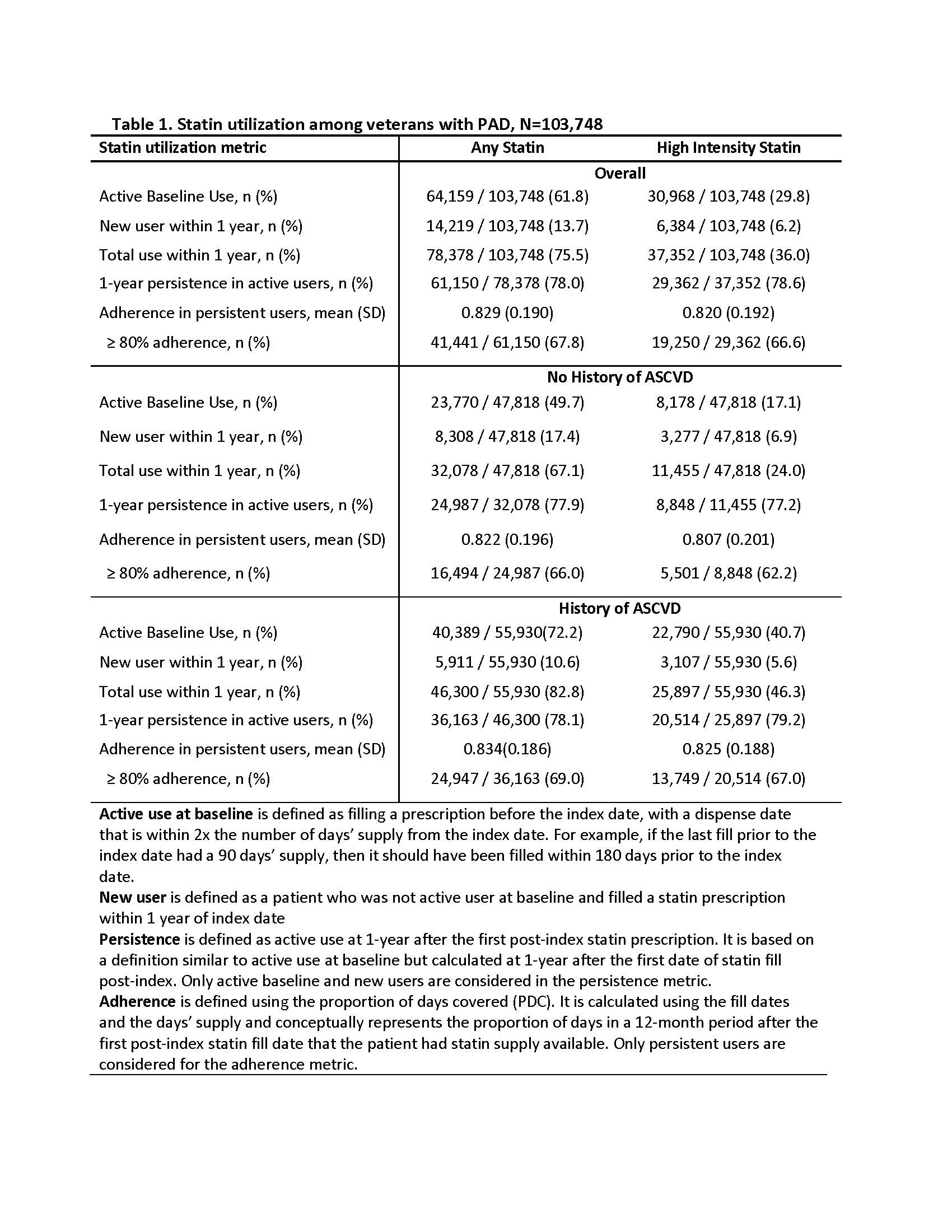
Methods: Using a novel natural language processing system that we previously developed and validated for identifying PAD, we created a registry of patients with a new diagnosis of PAD during 2015-2020 in the Veterans Health Administration (VHA). We used VHA pharmacy data to determine rates of active statin use at index date (date of PAD diagnosis), new initiation, persistence, and adherence (definitions in Table 1). High-intensity statin was based on statin type and dose. Analyses were also stratified by presence of pre-existing atherosclerotic cardiovascular disease (ASCVD), which was defined as a prior history of coronary or cerebrovascular disease.
Results: Among 103,748 patients, 64,159 (61.8%) were on statins at index date (baseline) and 30,968 (29.8%) were on high-intensity statins (Table 1). An additional 14,219 patients (13.7%) initiated statins within 1 year of PAD diagnosis, of whom 6,384 (6.2%) initiated high-intensity statins. Rates of active baseline use, or new initiation in 1 year were much lower in patients who did not have pre-existing ASCVD, compared to those with ASCVD (Table 1). Persistence with therapy among total active users was 78.0% for any statin and 78.6% for high-intensity statins. Mean adherence among persistent users was >80% for any statin and high intensity statin. Persistence and adherence did not differ in subgroups stratified by ASCVD.
Conclusion: Although more than 75% PAD patients were already on, or initiated a statin within 1 year of PAD diagnosis, only 36% were on high-intensity statins. Baseline use and new initiation of any statin and high-intensity statin was lower in PAD patients who did not have co-existing ASCVD. Overall, persistence among active users, and adherence among persistent users was high, and did not differ by statin intensity or pre-existing ASCVD. Given the strong evidence supporting high-intensity statins in PAD, interventions targeting intensifying statin therapy in PAD, especially in those without pre-existing ASCVD are warranted.
- Girotra, Saket ( University of Texas Southwestern , Dallas , Texas , United States )
- Gobbel, Glenn ( Vanderbilt University , Nashville , Tennessee , United States )
- Al-garadi, Mohammed ( Vanderbilt University , Nashville , Tennessee , United States )
- Smolderen, Kim ( Yale University , New Haven , Connecticut , United States )
- Arya, Shipra ( Stanford University , Palo Alto , California , United States )
- Beckman, Joshua ( UT Southwestern , Dallas , Texas , United States )
- Lund, Brian ( VA Health Care System , Iowa City , Iowa , United States )
- Li, Qiang ( University of Texas Southwestern , Dallas , Texas , United States )
- Vaughan Sarrazin, Mary ( University of Iowa , Iowa City , Iowa , United States )
- Nathani, Rohit ( University of Texas Southwestern Medical Center , Dallas , Texas , United States )
- Chan, Paul ( MID AMERICA HEART INSTITUTE , Kansas City , Missouri , United States )
- Nguyen, Cathy ( University of Texas Southwestern , Dallas , Texas , United States )
- Hoffman, Richard ( University of Iowa , Iowa City , Iowa , United States )
- Minniefield-young, Nicole ( North Texas Veterans Affairs Medical Center , Dallas , Texas , United States )
- Tsai, Shirling ( North Texas Veterans Affairs Medical Center , Dallas , Texas , United States )
Meeting Info:
Session Info:
Of Life And Limb: Peripheral Artery Disease
Sunday, 11/17/2024 , 03:15PM - 04:30PM
Moderated Digital Poster Session
More abstracts on this topic:
Jackson Elizabeth, Leblanc Erin, Haring Bernhard, Harrington Laura, Allison Matthew, Eaton Charles, Lamonte Michael, Hovey Kathleen, Andrews Chris, Wells Gretchen, Manson Joann, Levitan Emily, Spracklen Cassandra, Wild Robert
3D spheroids composed by induced Skeletal Muscle Progenitor Cells and Mesenchymal Stem Cells derived from human Pluripotent Stem Cells can recapitulate embryonic niches in hindlimb ischemia modelKim Jinju, Park Jae-hyun, Choi Yeon-jik, Park Hun
More abstracts from these authors:
Somisetty Medha, James Feroz, Vaughan Sarrazin Mary, Li Qiang, Nguyen Cathy, Nathani Rohit, Girotra Saket
Association Between Blood Pressure and Clinical Events in Peripheral Artery Disease with Small Vessel DiseaseNathani Rohit, Girotra Saket, Vaughan Sarrazin Mary, Chan Paul, Gupta Ajay, Kumbhani Dharam, Li Qiang, Nguyen Cathy, De Lemos James, Beckman Joshua