Final ID: 4139186
Bempedoic Acid and Limb Outcomes in Statin-Intolerant Patients with Peripheral Artery Disease
Abstract Body (Do not enter title and authors here): Introduction: Patients with peripheral artery disease (PAD) are at high risk of major adverse limb events (MALE) and CV events and have high rates of recurrence of these events. Recently, bempedoic acid was shown to reduce MACE in primary and secondary prevention patients. Whether bempedoic acid reduces the risk of MALE is unknown.
Methods: CLEAR Outcomes randomized 13,970 patients to bempedoic acid 180 mg or placebo. A clinical history of PAD was reported by investigators at baseline. Two blinded vascular medicine specialists independently adjudicated all adverse events for limb outcomes including worsening PAD symptoms leading to revascularization, chronic limb threatening ischemia, and acute limb ischemia events with the composite defined as MALE. Outcomes were assessed as time to first event as well as total (including recurrent) events using a negative binomial approach.
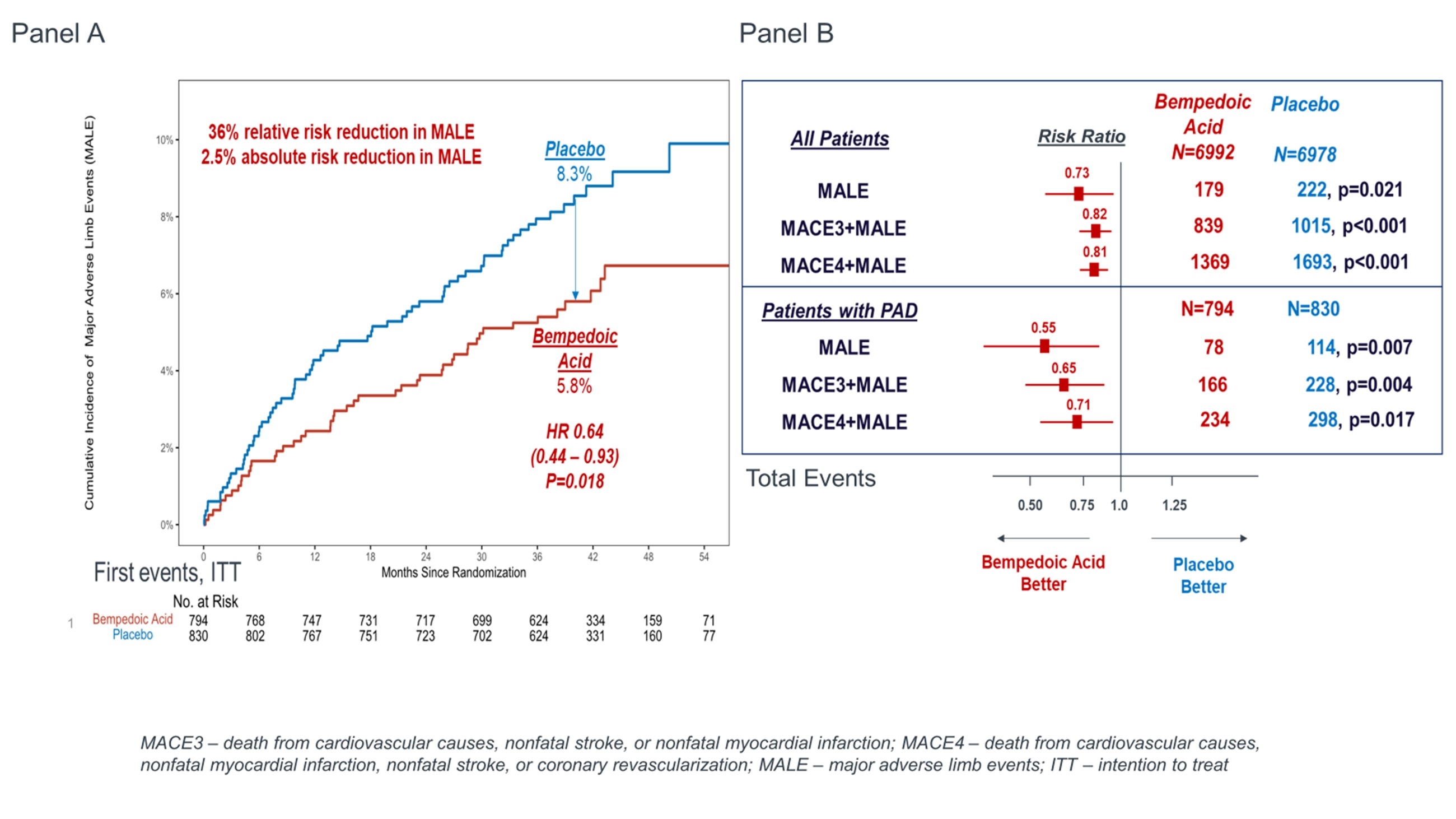
Results: A total of 1,624 of the enrolled patients had PAD at baseline. Over a median of 41.1 months the rate of first and total MALE events in patients with PAD in the placebo group were 8.3% and 13.7% respectively. Bempedoic acid reduced first MALE events by 36% (HR 0.64, 95% CI 0.44-0.93) with an absolute risk reduction (ARR) of 2.5% and number needed to treat (NNT) of 40, compared to placebo (figure, panel A). Similarly, when considering total events, bempedoic acid reduced MALE by 45% (RR 0.55, 95% CI 0.35 – 0.85, figure panel b). Consistent benefits were observed for MALE in the overall population as well as composites of MACE and MALE in both populations (Figure panel b). There was no evidence of effect modification for the benefit of bempedoic acid for MALE on the basis of PAD at baseline (p-interaction 0.09).
Conclusions: Bempedoic acid significantly reduces MALE. Benefits appear potentially greater when considering total (i.e. recurrent) events. These findings support the importance of LDL-C lowering therapy in patients with PAD. In addition, these findings support the early use of therapies with proven MALE benefit, including bempedoic acid, to optimize outcomes in PAD.
Methods: CLEAR Outcomes randomized 13,970 patients to bempedoic acid 180 mg or placebo. A clinical history of PAD was reported by investigators at baseline. Two blinded vascular medicine specialists independently adjudicated all adverse events for limb outcomes including worsening PAD symptoms leading to revascularization, chronic limb threatening ischemia, and acute limb ischemia events with the composite defined as MALE. Outcomes were assessed as time to first event as well as total (including recurrent) events using a negative binomial approach.
Results: A total of 1,624 of the enrolled patients had PAD at baseline. Over a median of 41.1 months the rate of first and total MALE events in patients with PAD in the placebo group were 8.3% and 13.7% respectively. Bempedoic acid reduced first MALE events by 36% (HR 0.64, 95% CI 0.44-0.93) with an absolute risk reduction (ARR) of 2.5% and number needed to treat (NNT) of 40, compared to placebo (figure, panel A). Similarly, when considering total events, bempedoic acid reduced MALE by 45% (RR 0.55, 95% CI 0.35 – 0.85, figure panel b). Consistent benefits were observed for MALE in the overall population as well as composites of MACE and MALE in both populations (Figure panel b). There was no evidence of effect modification for the benefit of bempedoic acid for MALE on the basis of PAD at baseline (p-interaction 0.09).
Conclusions: Bempedoic acid significantly reduces MALE. Benefits appear potentially greater when considering total (i.e. recurrent) events. These findings support the importance of LDL-C lowering therapy in patients with PAD. In addition, these findings support the early use of therapies with proven MALE benefit, including bempedoic acid, to optimize outcomes in PAD.
More abstracts on this topic:
Advanced Lipid Status Parameters in Women With Preeclampsia
Gojkovic Tamara, Saric Matutinovic Marija, Ivanisevic Jasmina, Vladimirov Sandra, Spasojevic Kalimanovska Vesna, Mikovic Zeljko, Stefanovic Aleksandra, Ardalic Daniela, Antonic Tamara, Banjac Gorica, Zeljkovic Aleksandra, Vekic Jelena, Miljkovic Trailovic Milica, Munjas Jelena, Jovicic Snezana
A Health Coach-Based Multi-Level Personalized Strategy Lowers LDL-Cholesterol and Enhances Lipid Control in Veterans with Atherosclerotic Cardiovascular Disease – The VA Lipid Optimization Reimagined Quality Improvement Project at VA New York Harbor Healthcare SystemChen Tina, Ingerman Diana, Haley Leah, Salovaara Priscilla, Nicholson Andrew, Illenberger Nicholas, Natarajan Sundar