Final ID: Su2192
Pharmacokinetics and pharmacodynamics of SC furosemide in patients with HF and obesity
Abstract Body (Do not enter title and authors here): Background
Obesity is increasingly prevalent in patients with heart failure (HF). Subcutaneous (SC) furosemide (Furoscix) is available as a 5-hour SC infusion via an on-body delivery device for the management of congestion, independent of ejection fraction. In a PK/PD study, the average bioavailability of Furoscix across the BMI spectrum was 99.6% with comparable diuresis and natriuresis to IV furosemide. However, the potential effects of obesity on the absorption and diuretic efficacy of Furoscix remain unknown.
Goal
To evaluate PK/PD of SC furosemide compared to IV administration, stratified by BMI.
Methods
Post-hoc analysis of SC furosemide pivotal PK/PD study was conducted to compare PK and urine output (UO) to IV furosemide, stratified by BMI. SC furosemide 80mg was administered over 5 hours per the typical biphasic administration schedule. IV furosemide was given as two 40mg injections, 2 hours apart. Plasma was collected to determine furosemide concentrations and UO was quantified over 8 hours. Descriptive statistics were reported.
Results
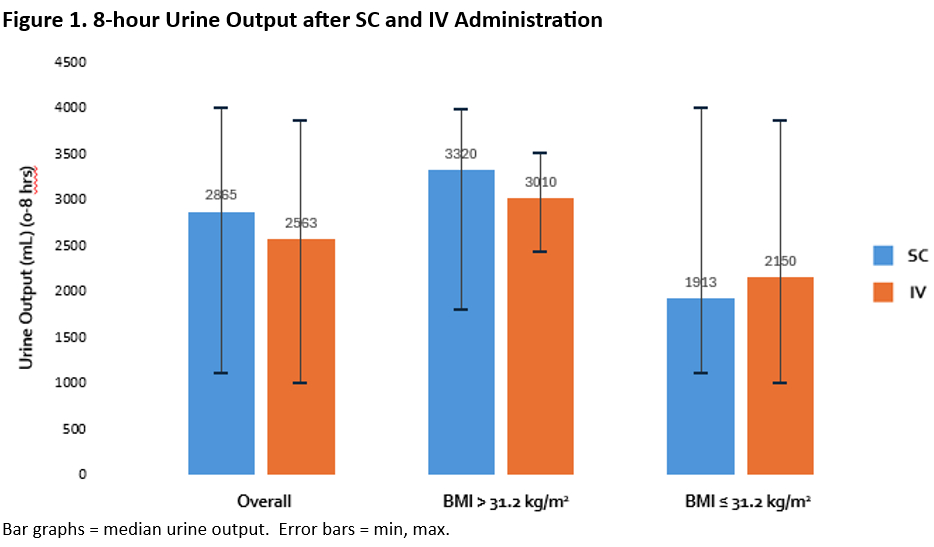
Sixteen patients were included for UO and 15 for the PK analysis. Median BMI was 31.2kg/m2; PK stratified by BMI is listed in Table 1. Peak plasma furosemide concentration (Cmax) was lower in the BMI >31.2kg/m2 group with no difference in t1/2 and greater UO at 8 hours in this cohort. There was no difference in any of the variables between the SC and IV agents across the BMI spectrum.
Conclusion
Although Furosemide Cmax was lower in the cohort with BMI >31.2kg/m2, total UO at 8 hours was higher. These findings were similar for the SC and IV formulations. Our data suggests that SC furosemide absorbs independent of BMI and provokes a reliable clinical response as measured by UO. Importantly, PK and UO results are consistently similar between the Furoscix and IV formulation across the BMI spectrum. Future research is warranted to investigate the reasons behind the increased UO in the population with higher BMI.
Obesity is increasingly prevalent in patients with heart failure (HF). Subcutaneous (SC) furosemide (Furoscix) is available as a 5-hour SC infusion via an on-body delivery device for the management of congestion, independent of ejection fraction. In a PK/PD study, the average bioavailability of Furoscix across the BMI spectrum was 99.6% with comparable diuresis and natriuresis to IV furosemide. However, the potential effects of obesity on the absorption and diuretic efficacy of Furoscix remain unknown.
Goal
To evaluate PK/PD of SC furosemide compared to IV administration, stratified by BMI.
Methods
Post-hoc analysis of SC furosemide pivotal PK/PD study was conducted to compare PK and urine output (UO) to IV furosemide, stratified by BMI. SC furosemide 80mg was administered over 5 hours per the typical biphasic administration schedule. IV furosemide was given as two 40mg injections, 2 hours apart. Plasma was collected to determine furosemide concentrations and UO was quantified over 8 hours. Descriptive statistics were reported.
Results
Sixteen patients were included for UO and 15 for the PK analysis. Median BMI was 31.2kg/m2; PK stratified by BMI is listed in Table 1. Peak plasma furosemide concentration (Cmax) was lower in the BMI >31.2kg/m2 group with no difference in t1/2 and greater UO at 8 hours in this cohort. There was no difference in any of the variables between the SC and IV agents across the BMI spectrum.
Conclusion
Although Furosemide Cmax was lower in the cohort with BMI >31.2kg/m2, total UO at 8 hours was higher. These findings were similar for the SC and IV formulations. Our data suggests that SC furosemide absorbs independent of BMI and provokes a reliable clinical response as measured by UO. Importantly, PK and UO results are consistently similar between the Furoscix and IV formulation across the BMI spectrum. Future research is warranted to investigate the reasons behind the increased UO in the population with higher BMI.
More abstracts on this topic:
Adipose tissue extracellular vesicles mediate pro-arrhythmic changes in atrial cardiomyocytes
Limpitikul Worawan, Garcia Contreras Marta, Betti Michael, Sheng Quanhu, Xiao Ling, Chatterjee Emeli, Gamazon Eric, Shah Ravi, Das Saumya
Biomarker and Edema Attenuation in IntraCerebral Hemorrhage (BEACH) (NCT05020535)Mould W Andrew, Thompson Richard, Hanley Daniel, Sansing Lauren, Ziai Wendy, Van Eldik Linda, Lane Karen, Whisler Brady Cailin, Kildahl Kaley, Walborn Nathan, Trivedi Pranshu Paresh, Mcbee Nichol, Economas Tracey, Carhuapoma Lourdes