Final ID: Su2162
The relationships between circulating biomarkers of iron deficiency and outcomes in patients with heart failure and reduced ejection fraction: insights from DAPA-HF.
Abstract Body (Do not enter title and authors here): Background: In patients with HFrEF, the guideline definition of iron deficiency (ID) uses both ferritin and transferrin saturation (TSAT) levels. However, observational studies and subgroup analyses of randomized controlled trials show that TSAT alone, or alternative biomarkers of ID, may better predict mortality and response to intravenous (iv) iron.
Goal: Examine the relationships between circulating biomarkers and alternative ID definitions and outcomes in patients with HFrEF in DAPA-HF.
Methods: Key inclusion criteria were: 1) NYHA class II-IV, 2) LVEF ≤40%, 3) elevated plasma NT-proBNP and 4) eGFR ≥30 ml/min/1.73m2. The primary outcome was a composite of a worsening HF event (HF hospitalization or an urgent HF visit requiring iv therapy) or cardiovascular death. Iron biomarkers were measured before randomization. The current guideline-recommended definition of ID is ferritin <100ng/mL or TSAT <20% and ferritin 100–299ng/mL. Alternative definitions examined were: iron ≤13umol/L, TSAT<20% (regardless of ferritin), and serum transferrin receptor (sTFR) >4.70mg/L (males) and >4.59mg/L (females).
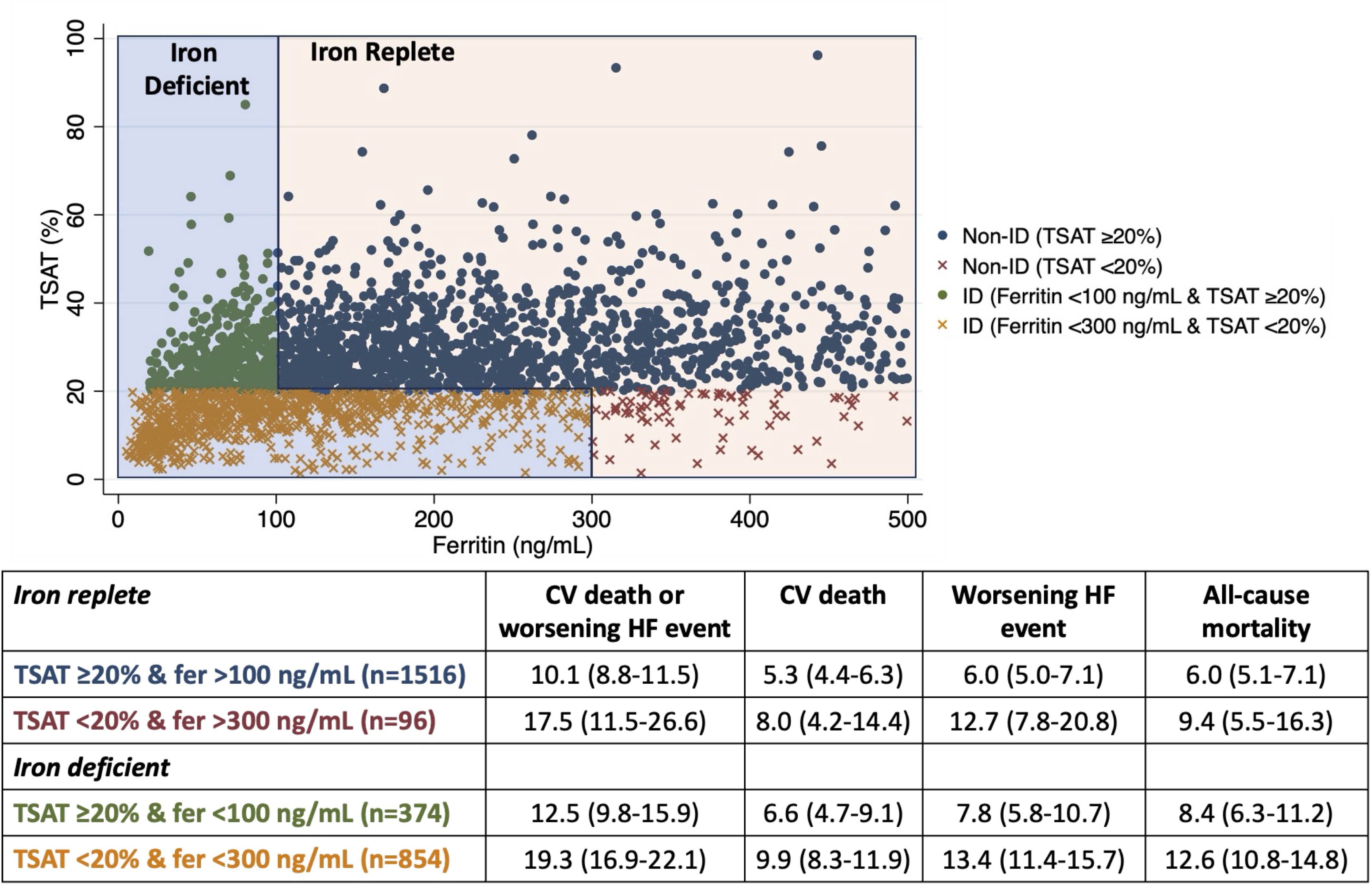
Results: Of the 4744 patients randomized, 2840 (60%) had ferritin, TSAT, iron and sTFR measured at baseline. Of these, 1228 (43%) had guideline-defined ID (374 [30%] based on ferritin <100ng/mL alone); 857 (70%) had ferritin <100ng/mL, 854 (70%) TSAT <20%, 853 (69%) iron ≤13umol/L, and 312 (25%) elevated sTFR. The lowest event rates were in iron replete patients with TSAT ≥20% and ferritin ≥100ng/mL (primary outcome=10.1/100 person-years [PY]) - Table. Event rates in iron replete patients with TSAT <20% and ferritin ≥300ng/mL were higher (17.5/100PY) and comparable to guideline-defined ID with TSAT <20% (19.3/100PY). Event rates were intermediary in guideline-defined ID patients with TSAT ≥20% and ferritin <100ng/mL (12.5/100 PY). The risk of the primary composite outcome per log-unit increase was: ferritin=HR 0.88, 95%CI 0.80-0.97; TSAT=0.75, 0.65-0.88; iron=0.79, 0.64-0.97 (and per log-unit decrease in sTFR=0.61, 0.48-0.76).
Conclusion: Low transferrin saturation is a predictor of adverse outcomes in HFrEF, irrespective of ferritin level.
Goal: Examine the relationships between circulating biomarkers and alternative ID definitions and outcomes in patients with HFrEF in DAPA-HF.
Methods: Key inclusion criteria were: 1) NYHA class II-IV, 2) LVEF ≤40%, 3) elevated plasma NT-proBNP and 4) eGFR ≥30 ml/min/1.73m2. The primary outcome was a composite of a worsening HF event (HF hospitalization or an urgent HF visit requiring iv therapy) or cardiovascular death. Iron biomarkers were measured before randomization. The current guideline-recommended definition of ID is ferritin <100ng/mL or TSAT <20% and ferritin 100–299ng/mL. Alternative definitions examined were: iron ≤13umol/L, TSAT<20% (regardless of ferritin), and serum transferrin receptor (sTFR) >4.70mg/L (males) and >4.59mg/L (females).
Results: Of the 4744 patients randomized, 2840 (60%) had ferritin, TSAT, iron and sTFR measured at baseline. Of these, 1228 (43%) had guideline-defined ID (374 [30%] based on ferritin <100ng/mL alone); 857 (70%) had ferritin <100ng/mL, 854 (70%) TSAT <20%, 853 (69%) iron ≤13umol/L, and 312 (25%) elevated sTFR. The lowest event rates were in iron replete patients with TSAT ≥20% and ferritin ≥100ng/mL (primary outcome=10.1/100 person-years [PY]) - Table. Event rates in iron replete patients with TSAT <20% and ferritin ≥300ng/mL were higher (17.5/100PY) and comparable to guideline-defined ID with TSAT <20% (19.3/100PY). Event rates were intermediary in guideline-defined ID patients with TSAT ≥20% and ferritin <100ng/mL (12.5/100 PY). The risk of the primary composite outcome per log-unit increase was: ferritin=HR 0.88, 95%CI 0.80-0.97; TSAT=0.75, 0.65-0.88; iron=0.79, 0.64-0.97 (and per log-unit decrease in sTFR=0.61, 0.48-0.76).
Conclusion: Low transferrin saturation is a predictor of adverse outcomes in HFrEF, irrespective of ferritin level.
More abstracts on this topic:
A Contemporary Machine Learning-Based Risk Stratification for Mortality and Hospitalization in Heart Failure with Preserved Ejection Fraction Using Multimodal Real-World Data
Fudim Marat, Weerts Jerremy, Patel Manesh, Balu Suresh, Hintze Bradley, Torres Francisco, Micsinai Balan Mariann, Rigolli Marzia, Kessler Paul, Touzot Maxime, Lund Lars, Van Empel Vanessa, Pradhan Aruna, Butler Javed, Zehnder Tobias, Sauty Benoit, Esposito Christian, Balazard Félix, Mayer Imke, Hallal Mohammad, Loiseau Nicolas
A validated metabolite-based biomarker score for fruit and vegetable intake and associations with all-cause mortality and incident cardiometabolic diseasesOude Griep Linda, Li Chunxiao, Koulman Albert, Imamura Fumiaki, Wareham Nicholas, Forouhi Nita