Final ID: MDP683
Lipid Profiles and Prognosis in Heart Failure: A Participant-Level Pooled Analysis of the PARADIGM-HF and PARAGON-HF Trials
AIM: To investigate classical (TC, LDL-C, triglycerides (TG), and HDL-C) and novel (RC) lipid parameters (LP) as risk determinants for CV events in HF patients across the LVEF spectrum.
METHODS: We pooled participant-level data from the PARADIGM-HF (LVEF ≤40%) and PARAGON-HF (LVEF ≥45%) trials to assess the association of LP with CV outcomes. RC was calculated as TC minus the sum of HDL-C and LDL-C. Lipid levels were classified per the National Lipid Association and RC by quartiles. The associations with time to first HF hospitalization and myocardial infarction (MI) were evaluated by adjusted Cox proportional hazards models stratified by trial. Restricted cubic splines accounted for potentially non-linear relationships.
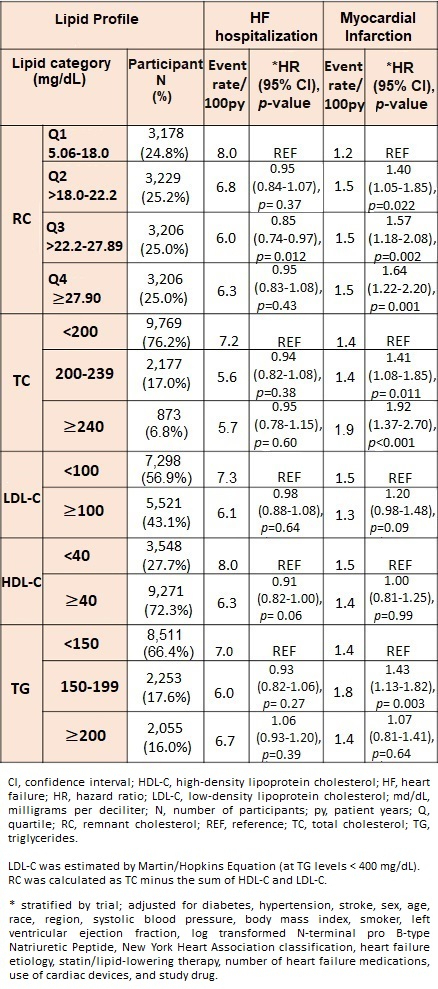
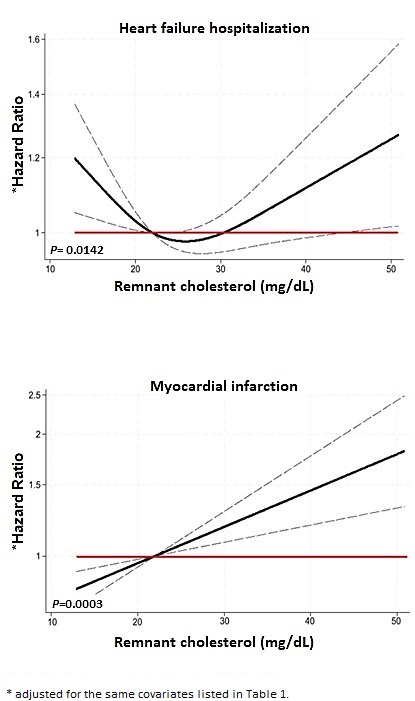
RESULTS: Of 12,819 participants with available lipid data, 1,967 (15.3%) experienced HF hospitalization and 442 (3.4%) had an MI. Over a median follow-up of 2.6 years, various LP were not consistently or significantly associated with risk of HF hospitalization, while higher RC and TC were linked to increased risk of MI (Table 1). When evaluating LP continuously, there was a significant non-linear association between RC and risk of HF hospitalization, with a nadir in risk at ~ 20-30 mg/dL (Figure 1). In contrast, higher levels of RC, LDL-C, and TC were linearly and significantly associated with risk of MI. None of the results were modified by LVEF.
CONCLUSION: In a global HF population, higher RC levels were consistently associated with increased risk of MI, while the relationship between RC and the more frequent endpoint of HF hospitalization appeared to be more complex and inconsistent. These data might explain why previous interventional lipid lowering trials have not modified disease course in HF.
- Siqueira, Sara ( Brigham and Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Jhund, Pardeep ( University of Glasgow , Glasgow , United Kingdom )
- Mcmurray, John ( University of Glasgow , Glasgow , United Kingdom )
- Vaduganathan, Muthiah ( Brigham and Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Solomon, Scott ( Brigham and Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Pabon, Maria ( Brigham and Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Claggett, Brian ( Brigham and Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Packer, Milton ( Baylor University Medical Center , Dallas , Texas , United States )
- Lam, Carolyn ( Duke-NUS Medical School , Singapore , Singapore )
- Rouleau, Jean ( Institut de Cardiologie de Montréal, Université de Montréal , Montreal , Quebec , Canada )
- Zile, Michael ( Medical University of South Carolina , Charleston , South Carolina , United States )
- Lefkowitz, Marty ( Novartis Pharmaceuticals Corporation , East Hanover , New Jersey , United States )
- Desai, Akshay ( Brigham and Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
Meeting Info:
Session Info:
Cardiometabolic Conundrums in Heart Failure
Saturday, 11/16/2024 , 11:10AM - 12:25PM
Moderated Digital Poster Session
More abstracts on this topic:
Marway Prabhvir, Campello Jorge Carlos Alberto, Wagner Catherine, Baker Timothy, Burris Nicholas
A Cardiac Targeting Peptide Linked to miRNA106a Targets and Suppresses Genes Known to Cause Heart Failure: Reversing Heart Failure at the SourceLu Ming, Deng Claire, Taskintuna Kaan, Ahern Gerard, Yurko Ray, Islam Kazi, Zahid Maliha, Gallicano Ian
More abstracts from these authors:
Ostrominski John, Pitt Bertram, Zannad Faiez, Zile Michael, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Lassen Mats, Butt Jawad, Claggett Brian, Anand Inder, Desai Akshay, Jhund Pardeep, Lam Carolyn, Pfeffer Marc
Age, Adiposity-Related Anthropometrics, and Clinical Outcomes in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: A Participant-Level Pooled Analysis of Randomized TrialsOstrominski John, Pitt Bertram, Zannad Faiez, Zile Michael, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Lassen Mats, Butt Jawad, Claggett Brian, Anand Inder, Desai Akshay, Jhund Pardeep, Lam Carolyn, Pfeffer Marc