Final ID: Sa1043
Anti-atherosclerotic Potential of M2 Macrophage-Derived Exosomes by Promoting Cholesterol Efflux of Foam Cell-Like Vascular Smooth Muscle Cells via the PPARγ-LXRα-ABCA1/ ABCG1 Pathway
Abstract Body (Do not enter title and authors here): Background: Impediment in the excretion of lipid deposits within SMC-derived foam cells (SMC-FCs) is one of the reasons for the continuous expansion of the plaque necrotic core. This study aims to explore the effect and underlying mechanimsm of exosomes secreted by M2 macrophage (M2-exos) on SMC-FCs lipid metabolism and plaque stability.
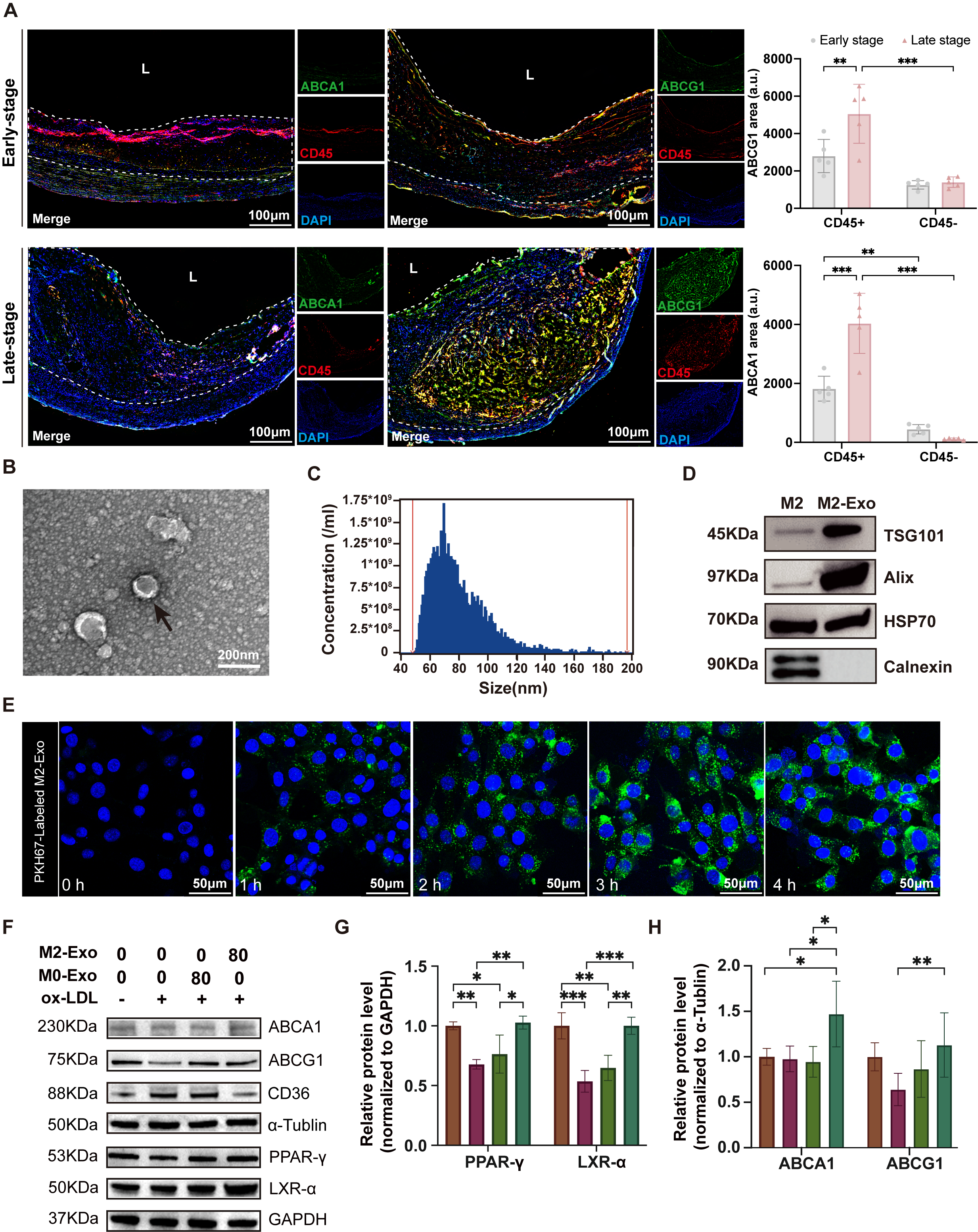
Methods: First, immunofluorescence was used to detect the expression levels of CD45 (a recognized differentially expressed molecule of myeloid and SMC-FCs) and the key proteins of cholesterol efflux pathway, ABCA1 and ABCG1, in human early and late plaques. Exosomes derived from M0 and M2 macrophages were purified by sucrose density gradient centrifugation, and characterized based on specific morphology and surface markers. Western blot, Oil red O staining and cell total cholesterol assay were used to assess the effects of M2-exos on the lipid mechanism of SMC-FCs. RNA-seq was used to detect the miRNA profiles of M2-exos. Quantitative real-time PCR was used to identify candidate miRNAs. The dual-luciferase reporting system was utilized to assess the regulatory effect of candidate miRNA on target gene. Then, the effect of M2-exos on the progression and stability of plaques in ApoE-/- mice was evaluated using Oil red O, H&E, Masson, Movat and immunohistochemistry.
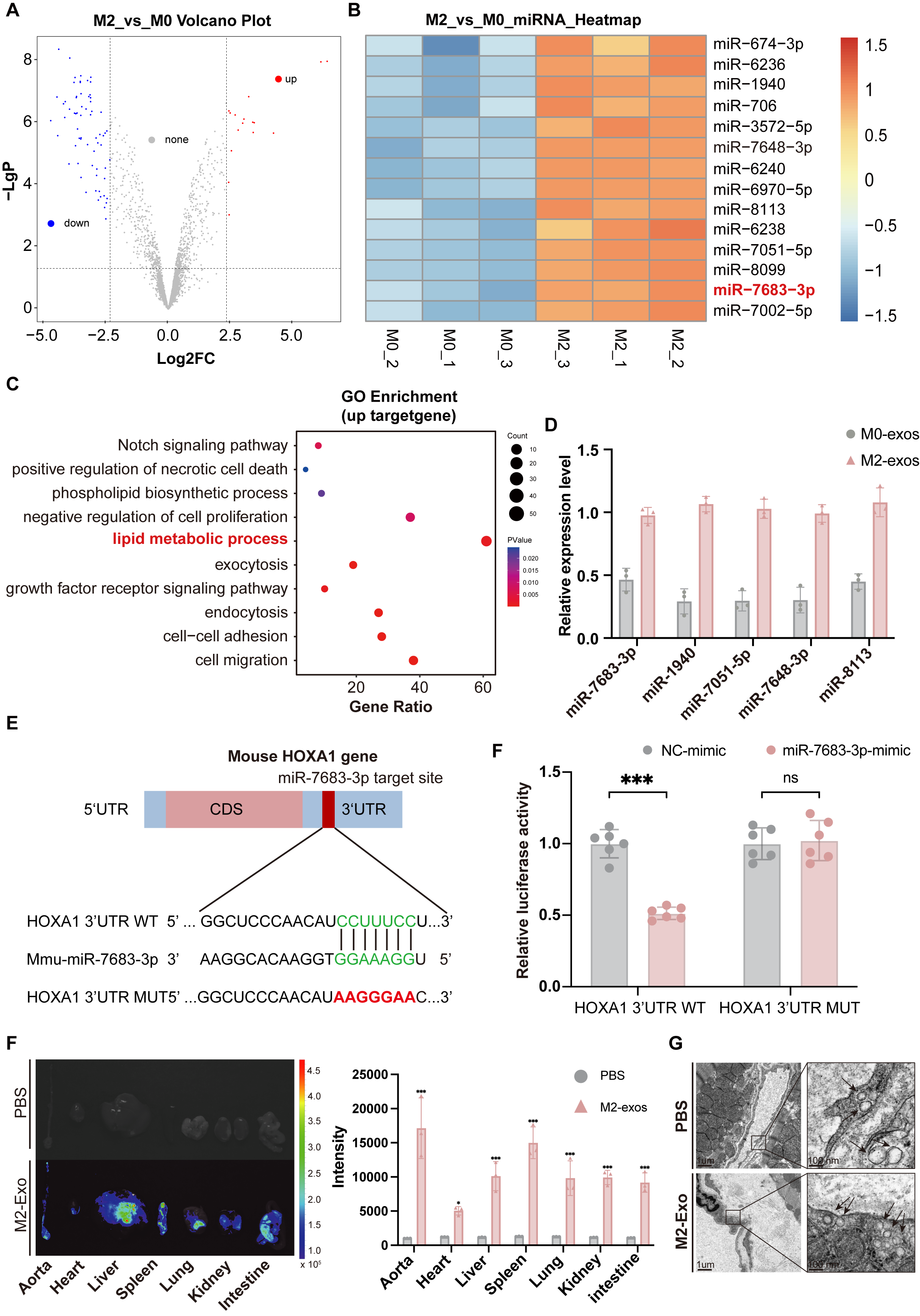
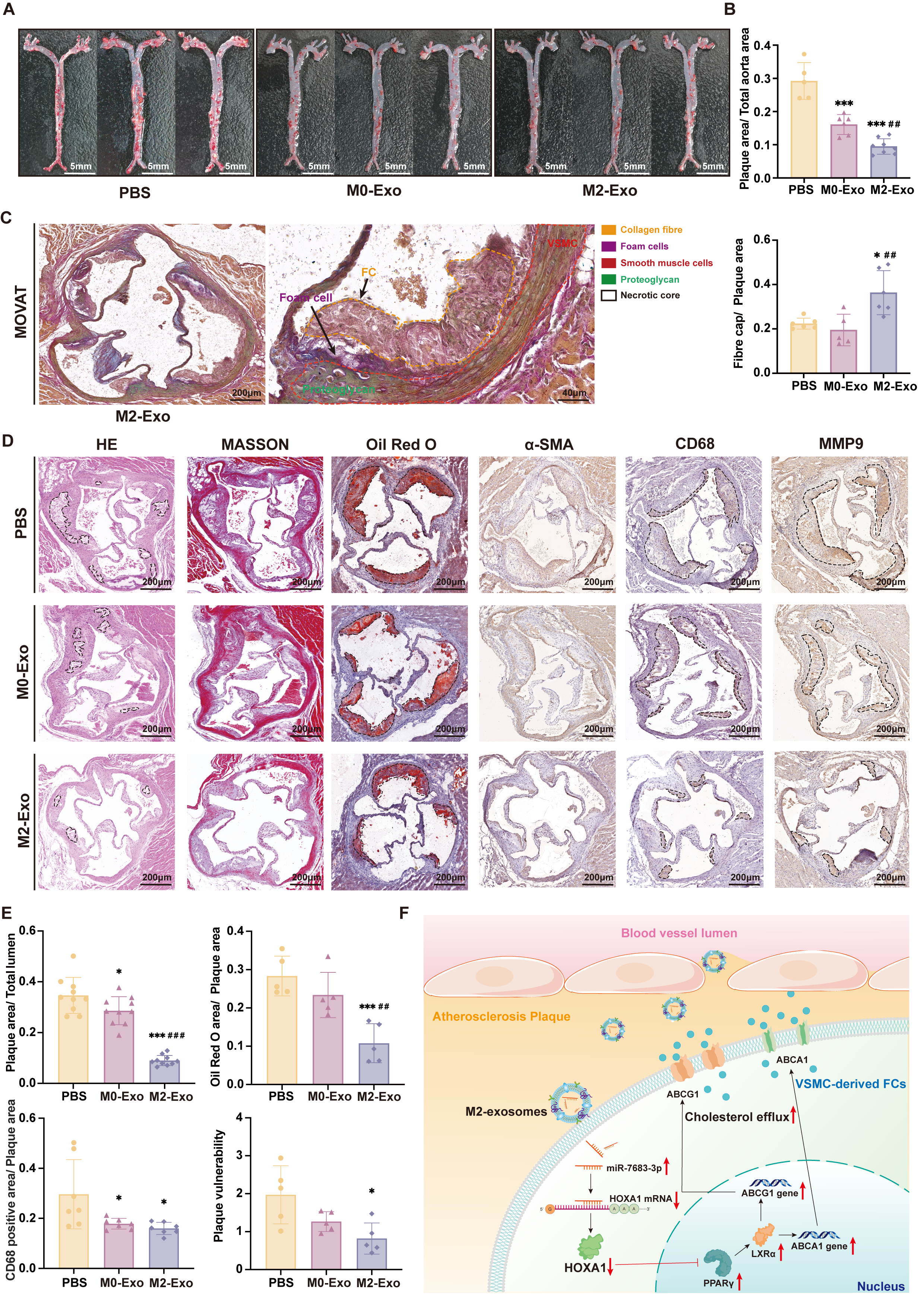
Results: Immunofluorescence revealed that compared with early plaques, VSMC-FCs (CD45-) were significantly increased in late plaques, and the expression levels of ABCG1 and ABCA1 were marked lower than those in macrophage-derived foam cells (CD45+). Purified M2-exos treatment significantly promoted the cholesterol efflux of SMC-FCs in vitro. In high-fat-fed ApoE-/-mice, M2-exos significantly reduced the VSMC-FCs, delayed the progression of plaques, decreased necrotic core area and enhanced plaque stability. MiRNA profiling and comprehensive analysis of signaling pathways showed that M2-exos were rich in miR-7683-3p, which played a key role in regulating SMC-FCs lipid metabolism through PPARγ-LXRα-ABCA1/ABCG1 pathway. Dual-luciferase reporting assay showed that miR-7683-3p can specifically bind to the promoter region of homeobox genes A1(HoxA1), an inhibitor of PPARγ-LXRα-ABCA1/ABCG1 pathway.
Conclusion: M2-exos exerted an obvious atherosclerotic protective effect, and the underlying mechanism was closely related to MiR-7683-3p, which targeted the 3’UTR of HoxA1 mRNA and activated the PPARγ-LXRα-ABCA1/ABCG1 mediated cholesterol efflux in SMC-FCs.
Methods: First, immunofluorescence was used to detect the expression levels of CD45 (a recognized differentially expressed molecule of myeloid and SMC-FCs) and the key proteins of cholesterol efflux pathway, ABCA1 and ABCG1, in human early and late plaques. Exosomes derived from M0 and M2 macrophages were purified by sucrose density gradient centrifugation, and characterized based on specific morphology and surface markers. Western blot, Oil red O staining and cell total cholesterol assay were used to assess the effects of M2-exos on the lipid mechanism of SMC-FCs. RNA-seq was used to detect the miRNA profiles of M2-exos. Quantitative real-time PCR was used to identify candidate miRNAs. The dual-luciferase reporting system was utilized to assess the regulatory effect of candidate miRNA on target gene. Then, the effect of M2-exos on the progression and stability of plaques in ApoE-/- mice was evaluated using Oil red O, H&E, Masson, Movat and immunohistochemistry.
Results: Immunofluorescence revealed that compared with early plaques, VSMC-FCs (CD45-) were significantly increased in late plaques, and the expression levels of ABCG1 and ABCA1 were marked lower than those in macrophage-derived foam cells (CD45+). Purified M2-exos treatment significantly promoted the cholesterol efflux of SMC-FCs in vitro. In high-fat-fed ApoE-/-mice, M2-exos significantly reduced the VSMC-FCs, delayed the progression of plaques, decreased necrotic core area and enhanced plaque stability. MiRNA profiling and comprehensive analysis of signaling pathways showed that M2-exos were rich in miR-7683-3p, which played a key role in regulating SMC-FCs lipid metabolism through PPARγ-LXRα-ABCA1/ABCG1 pathway. Dual-luciferase reporting assay showed that miR-7683-3p can specifically bind to the promoter region of homeobox genes A1(HoxA1), an inhibitor of PPARγ-LXRα-ABCA1/ABCG1 pathway.
Conclusion: M2-exos exerted an obvious atherosclerotic protective effect, and the underlying mechanism was closely related to MiR-7683-3p, which targeted the 3’UTR of HoxA1 mRNA and activated the PPARγ-LXRα-ABCA1/ABCG1 mediated cholesterol efflux in SMC-FCs.
More abstracts on this topic:
Impact of Oxidative Stress on Aortic Vulnerable Plaques Detected by Non-obstructive General Angioscopy
Kojima Keisuke, Okumura Yasuo, Tanaka Yudai, Mizobuchi Saki, Migita Shohei, Fukumoto Katsunori, Ebuchi Yasunari, Arai Riku, Murata Nobuhiro, Fukamachi Daisuke
Comprehensive Plasma Transcriptomics Profiling Identifies a Small Set of Circulating MicroRNA Biomarkers to Distinguish Hypertrophic Cardiomyopathy from Other Cardiomyopathies with Left Ventricular HypertrophyKiyohara Yuko, Akita Keitaro, Fifer Michael, Teruya Sergio, Bampatsias Dimitrios, Mirabal Alfonsina, Maurer Mathew, Shimada Yuichi