Scientific Sessions 2024
/
Heart Failure Potpourri
/
Defining Severe Heart Failure across the Ejection Fraction Spectrum in DAPA-HF and DELIVER
Final ID: Su1072
Defining Severe Heart Failure across the Ejection Fraction Spectrum in DAPA-HF and DELIVER
Abstract Body (Do not enter title and authors here): Background: Patients with severe heart failure (HF) experience debilitating clinical symptoms and worse cardiovascular (CV) outcomes with an excess mortality risk. Until recently, severe HF has largely been considered only in those with reduced ejection fraction. The ESC HFA has put forth a consensus definition for severe or advanced HF that may be applicable across the ejection fraction (EF) spectrum.
Hypothesis: Patients with severe HF experience a higher rate of CV outcomes. The safety and efficacy of SGLT2i dapagliflozin is consistent regardless of the severe HF status.
Aims: To assess the prevalence, CV outcome risk, and treatment response to the SGLT2i dapagliflozin among patients with severe HF.
Methods: DAPA-HF and DELIVER were randomized, double-blind trials, testing dapagliflozin vs placebo in 11,007 patients with symptomatic HF across the full spectrum of EF. Severe HF was adapted from the ESC HFA definition: NYHA class III/IV, evidence of HF with reduced, mildly reduced or preserved EF, elevated natriuretic peptides, HF hospitalization within previous 12 months, and adverse patient-reported symptom burden (KCCQ-TSS<75). Outcomes were assessed and the treatment effect of dapagliflozin was measured for the primary endpoint of CV death or first worsening HF event (including HF hospitalization and urgent HF visit) by severe HF status.
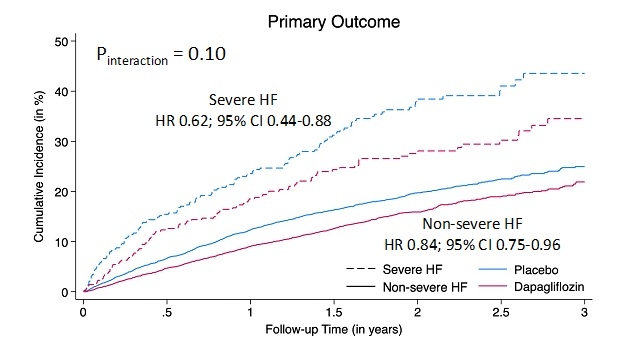
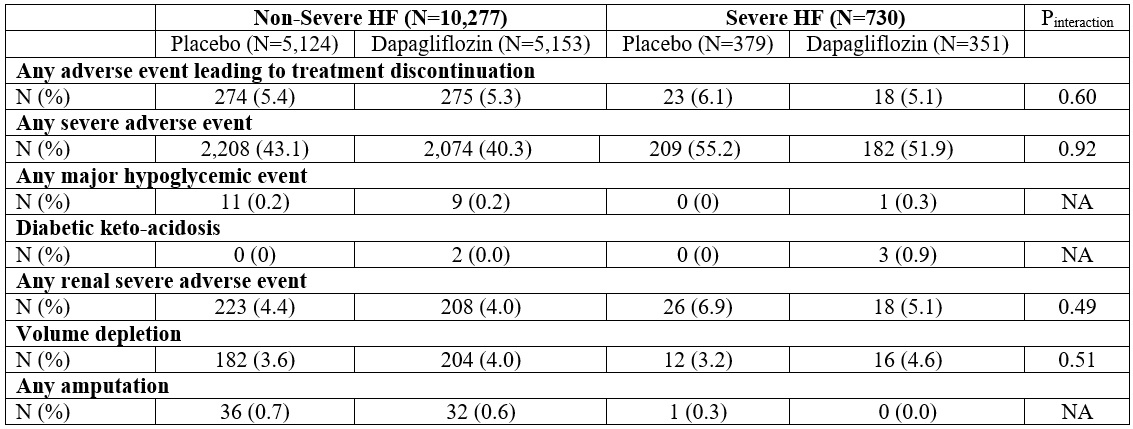
Results: Overall, 730 (6.6%) fulfilled the severe HF definition (296 [40.6%] with EF ≤40%, 192 [26.3%] with EF 41-49%, and 232 [33.2%] with EF ≥50%). Patients with severe HF had a higher comorbidity burden and higher NT-proBNP values. Over a median follow-up of 22.4 months, the primary endpoint occurred in 231 patients, at a rate of 20 (17-23) per 100py. Patients with severe HF experienced a higher rate of events compared to patients without severe HF (adjusted hazard ratio [HR] 1.44, 95% confidence interval [CI] 1.20-1.75), across the spectrum of LVEF (Pinteraction=0.85). Treatment with dapagliflozin was consistently beneficial in reducing the risk of the primary endpoint regardless of the severe HF status (Pinteraction=0.10, Figure 1). The safety profile of dapagliflozin was also consistent irrespective of the severe HF status (Figure 2).
Conclusions: In a large, global contemporary HF trial population, “severe HF” was associated with excess risk of CV events across the spectrum of EF. Treatment with the SGLT2i dapagliflozin appears safe and effective in reducing the risk of CV death or worsening HF in this population.
Hypothesis: Patients with severe HF experience a higher rate of CV outcomes. The safety and efficacy of SGLT2i dapagliflozin is consistent regardless of the severe HF status.
Aims: To assess the prevalence, CV outcome risk, and treatment response to the SGLT2i dapagliflozin among patients with severe HF.
Methods: DAPA-HF and DELIVER were randomized, double-blind trials, testing dapagliflozin vs placebo in 11,007 patients with symptomatic HF across the full spectrum of EF. Severe HF was adapted from the ESC HFA definition: NYHA class III/IV, evidence of HF with reduced, mildly reduced or preserved EF, elevated natriuretic peptides, HF hospitalization within previous 12 months, and adverse patient-reported symptom burden (KCCQ-TSS<75). Outcomes were assessed and the treatment effect of dapagliflozin was measured for the primary endpoint of CV death or first worsening HF event (including HF hospitalization and urgent HF visit) by severe HF status.
Results: Overall, 730 (6.6%) fulfilled the severe HF definition (296 [40.6%] with EF ≤40%, 192 [26.3%] with EF 41-49%, and 232 [33.2%] with EF ≥50%). Patients with severe HF had a higher comorbidity burden and higher NT-proBNP values. Over a median follow-up of 22.4 months, the primary endpoint occurred in 231 patients, at a rate of 20 (17-23) per 100py. Patients with severe HF experienced a higher rate of events compared to patients without severe HF (adjusted hazard ratio [HR] 1.44, 95% confidence interval [CI] 1.20-1.75), across the spectrum of LVEF (Pinteraction=0.85). Treatment with dapagliflozin was consistently beneficial in reducing the risk of the primary endpoint regardless of the severe HF status (Pinteraction=0.10, Figure 1). The safety profile of dapagliflozin was also consistent irrespective of the severe HF status (Figure 2).
Conclusions: In a large, global contemporary HF trial population, “severe HF” was associated with excess risk of CV events across the spectrum of EF. Treatment with the SGLT2i dapagliflozin appears safe and effective in reducing the risk of CV death or worsening HF in this population.
- Inciardi, Riccardo ( ASST Spedali Civili , Brescia , BS , Italy )
- De Boer, Rudolf ( University Medical Center Groningen , Groningen , Netherlands )
- Hernandez, Adrian ( Duke Clinical Research Institute , Durham , North Carolina , United States )
- Shah, Sanjiv ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Kober, Lars ( RIGSHOSPITALET , Copenhagen , Denmark )
- Ponikowski, Piotr ( Wroclaw Medical University , Wroclaw , Poland )
- Sabatine, Marc ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Petersson, Magnus ( AstraZeneca , Molndal , Sweden )
- Langkilde, Anna Maria ( ASTRAZENECA , Mölndal , Sweden )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Lu, Henri ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Jhund, Pardeep ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Lam, Carolyn ( NATIONAL HEART CENTRE SINGAPORE , Singapore , Singapore )
- Kosiborod, Mikhail ( ST LUKES HEALTH SYSTEM , Kansas City , Missouri , United States )
- Inzucchi, Silvio ( Yale University School of Medicine , New Haven , Connecticut , United States )
- Martinez, Felipe ( INSTITUTO DAMIC , Cordoba , Argentina )
Author Disclosures:
Riccardo Inciardi: DO NOT have relevant financial relationships
| Rudolf De Boer: No Answer
| Adrian Hernandez: DO have relevant financial relationships
;
Researcher:AstraZeneca:Active (exists now)
; Researcher:Verve:Active (exists now)
; Researcher:Intellia:Active (exists now)
; Researcher:NovoNordisk:Active (exists now)
; Researcher:Merck:Active (exists now)
; Researcher:Novartis:Active (exists now)
; Researcher:Cytokinetics:Active (exists now)
; Researcher:Amgen:Active (exists now)
; Researcher:Boehringer Ingelheim:Active (exists now)
; Researcher:Bayer:Active (exists now)
| Sanjiv Shah: DO have relevant financial relationships
;
Consultant:Bayer:Active (exists now)
; Consultant:Merck:Active (exists now)
; Consultant:Axon Therapies:Active (exists now)
; Consultant:Corvia :Active (exists now)
; Consultant:Boehringer-Ingelheim:Active (exists now)
; Consultant:Bristol-Myers Squibb:Active (exists now)
; Consultant:Ionis:Active (exists now)
; Consultant:Novartis:Active (exists now)
; Consultant:Tenax:Active (exists now)
; Consultant:Intellia:Active (exists now)
; Consultant:Rivus:Active (exists now)
; Consultant:Novo Nordisk:Active (exists now)
; Consultant:Lilly:Active (exists now)
; Consultant:Pfizer:Active (exists now)
; Consultant:AstraZeneca:Active (exists now)
| Lars Kober: DO have relevant financial relationships
;
Speaker:Astra Zeneca:Active (exists now)
; Speaker:Novartis:Active (exists now)
; Speaker:Novo:Active (exists now)
; Speaker:Boehringer:Active (exists now)
| Piotr Ponikowski: No Answer
| Marc Sabatine: DO have relevant financial relationships
;
Research Funding (PI or named investigator):Amgen:Active (exists now)
; Research Funding (PI or named investigator):Pfizer:Active (exists now)
; Research Funding (PI or named investigator):Novartis:Active (exists now)
; Research Funding (PI or named investigator):Merck:Active (exists now)
; Research Funding (PI or named investigator):Ionis:Active (exists now)
; Consultant:AstraZeneca:Active (exists now)
; Individual Stocks/Stock Options:AstraZeneca:Active (exists now)
; Consultant:Anthos:Active (exists now)
; Research Funding (PI or named investigator):Anthos:Active (exists now)
; Consultant:Amgen:Active (exists now)
| Magnus Petersson: DO have relevant financial relationships
;
Employee:AstraZeneca:Active (exists now)
; Individual Stocks/Stock Options:AstraZeneca:Active (exists now)
| Anna Maria Langkilde: DO have relevant financial relationships
;
Employee:AstraZeneca:Active (exists now)
; Individual Stocks/Stock Options:AstraZeneca:Active (exists now)
| John McMurray: DO have relevant financial relationships
;
Research Funding (PI or named investigator):British Heart Foundation, National Institute for Health – National Heart Lung and Blood Institute (NIH-NHLBI), Boehringer Ingelheim, SQ Innovations, Catalyze Group. - Payment to Glasgow University:Active (exists now)
; Other (please indicate in the box next to the company name):KBP Biosciences - My employer, Glasgow University, has been paid by KBP Biosciences for my time spent scientific advisor to company to help guide clinical development in cardio-renal disease, inflammation & infection.:Past (completed)
; Consultant:WIRB-Copernicus Group Clinical Inc. - Data Safety Monitoring Board:Active (exists now)
; Other (please indicate in the box next to the company name):Bayer - My employer, Glasgow University, has been paid by Bayer for my time spent as Steering Committee member for the PANACHE trial using neladenoson bialanate and for the FINEARTS trial with finerenone. These payments were made through a Consultancy with Glasgow University and I have not received personal payments in relation to these trials/drugs.:Active (exists now)
; Other (please indicate in the box next to the company name):Glaxo Smith Kline - My employer, Glasgow University, has been paid by GSK for my time spent as Co-principal investigator and Steering Committee member, respectively, for the Harmony-Outcomes trial (albiglutide) and two trials, ASCEND-D and ASCEND-ND, using daprodustat, and meetings related to these trials. GSK has also paid my travel and accommodation for some of these meetings. These payments were made through a Consultancy with Glasgow University and I have not received personal payments in relation to these trials/drugs.:Active (exists now)
; Other (please indicate in the box next to the company name):Novartis - My employer, Glasgow University, has been paid by Novartis for my time spent as Executive Committee member and then co-principal investigator of ATMOSPHERE, co-principal investigator of the PARADIGM-HF and PARAGON-HF trials and Executive/Steering Committee member for PARADISE-MI, PERSPECTIVE and PARACHUTE-HF trials (with sacubitril/valsartan) and meetings/presentations related to these trials and aliskiren and sacubitril/valsartan. Novartis has also paid my travel and accommodation for some of these meetings. These payments were made through a Consultancy with Glasgow University and I have not received personal payments from Novartis in relation to these trials/drugs.:Active (exists now)
; Other (please indicate in the box next to the company name):Cardurion - My employer, Glasgow University, has been paid by Cardurion for my participation in a company advisory board about development of a PDE 9 inhibitor in heart failure.:Active (exists now)
; Consultant:Global Clinical Trial Partners Ltd. - Director:Active (exists now)
; Consultant:Alynylam Pharmaceuticals, Amgen, AnaCardio, AstraZeneca, Bayer, Berlin Cures, BMS, Cardurion, Cytokinetics, Ionis Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, River 2 Renal Corp.:Active (exists now)
; Other (please indicate in the box next to the company name):Theracos - My employer, Glasgow University, has been paid by Theracos for my time spent as Principal investigator for the BEST trial and meetings related to this trial. Theracos has also paid my travel and accommodation for some of these meetings. These payments were made through a Consultancy with Glasgow University and I have not received personal payments in relation to this trial/this drug.:Past (completed)
; Other (please indicate in the box next to the company name):Amgen - My employer, Glasgow University, has been paid by Amgen for my time spent as Steering Committee member for the ATOMIC-HF, COSMIC-HF and GALACTIC-HF trials and meetings and other activities related to these trials. Amgen has also paid my travel and accommodation for some of these meetings/activities. These payments were made through a Consultancy with Glasgow University and I have not received personal payments in relation to these trials/this drug.:Active (exists now)
; Other (please indicate in the box next to the company name):Cytokinetics - My employer, Glasgow University, has been paid by Cytokinetics for my time spent as Steering Committee member for the ATOMIC-HF, COSMIC-HF and GALACTIC-HF trials and meetings and other activities related to these trials. Cytokinetics has also paid my travel and accommodation for some of these meetings/activities. These payments were made through a Consultancy with Glasgow University and I have not received personal payments in relation to these trials/this drug.:Active (exists now)
; Speaker:Abbott, Alkem Metabolics, Astra Zeneca, Blue Ocean Scientific Solutions Ltd., Boehringer Ingelheim, Canadian Medical and Surgical Knowledge, Emcure Pharmaceuticals Ltd., Eris Lifesciences, European Academy of CME, Hikma Pharmaceuticals, Imagica Health, Intas Pharmaceuticals, J.B. Chemicals & Pharmaceuticals Ltd., Lupin Pharmaceuticals, Medscape/Heart.Org., ProAdWise Communications, Radcliffe Cardiology, Sun Pharmaceuticals, The Corpus, Translation Research Group, Translational Medicine Academy - personal lecture fees:Active (exists now)
; Other (please indicate in the box next to the company name):AstraZeneca - My employer, Glasgow University, has been paid by AstraZeneca (who market dapagliflozin) for my time spent as Principal Investigator of DAPA-HF and Co-principal Investigator of DELIVER and DETERMINE (trials using dapagliflozin) in heart failure and meetings and other activities related to these trials. AstraZeneca has also paid my travel and accommodation for these meetings. These payments were made through a Consultancy with Glasgow University and I have not received personal payments in relation to this trial/this drug.:Active (exists now)
| Muthiah Vaduganathan: DO have relevant financial relationships
;
Researcher:received research grant support, served on advisory boards, or had speaker engagements with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, BMS, Boehringer Ingelheim, Chiesi, Cytokinetics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, and participates on clinical trial committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics:Active (exists now)
| Henri Lu: No Answer
| Scott Solomon: DO have relevant financial relationships
;
Research Funding (PI or named investigator):Alexion, Alnylam, Applied Therapeutics, AstraZeneca, Bellerophon, Bayer, BMS, Boston Scientific, Cytokinetics, Edgewise, Eidos/BridgeBio, Gossamer, GSK, Ionis, Lilly,NIH/NHLBI, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Tenaya, Theracos, US2.AI:Active (exists now)
; Consultant:Abbott, Action, Akros, Alexion, Alnylam, Amgen, Arena, AstraZeneca, Bayer, BMS, Cardior, Cardurion, Corvia, Cytokinetics, GSK, Intellia, Lilly, Novartis, Roche, Theracos, Quantum Genomics, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, Valo:Active (exists now)
| Brian Claggett: DO have relevant financial relationships
;
Consultant:Cardior:Active (exists now)
; Consultant:Eli Lilly:Active (exists now)
; Consultant:CVRx:Past (completed)
; Consultant:Intellia:Active (exists now)
; Consultant:Cytokinetics:Past (completed)
; Consultant:Cardurion:Active (exists now)
| Akshay Desai: DO have relevant financial relationships
;
Research Funding (PI or named investigator):Abbott:Active (exists now)
; Individual Stocks/Stock Options:DTX plus:Past (completed)
; Consultant:Veristat, Zydus:Past (completed)
; Consultant:Medpace, Porter Health, Regeneron, River2Renal, Roche, Verily:Active (exists now)
; Consultant:Merck, Medtronic, Parexel, scPharmaceuticals:Past (completed)
; Consultant:Bayer, Biofourmis, Novartis:Active (exists now)
; Consultant:Avidity, Axon Therapeutics, Boston Scientific, GlaxoSmithKline:Past (completed)
; Consultant:Abbott, Alnylam, AstraZeneca:Active (exists now)
; Research Funding (PI or named investigator):Pfizer:Active (exists now)
; Research Funding (PI or named investigator):Novartis:Active (exists now)
; Research Funding (PI or named investigator):Bayer:Active (exists now)
; Research Funding (PI or named investigator):AstraZeneca:Active (exists now)
; Research Funding (PI or named investigator):Alnylam:Active (exists now)
| Pardeep Jhund: DO have relevant financial relationships
;
Consultant:AstraZeneca:Active (exists now)
; Other (please indicate in the box next to the company name):Director GCTP :Active (exists now)
; Researcher:Analog Devices :Active (exists now)
; Researcher:Roche Diagnostics:Active (exists now)
; Researcher:AstraZeneca:Past (completed)
; Researcher:Boheringer Ingelheim:Past (completed)
; Consultant:Bayer:Active (exists now)
| Carolyn Lam: DO have relevant financial relationships
;
Consultant:Alleviant Pharma, Allysta Pharma:Active (exists now)
; Other (please indicate in the box next to the company name):Us2.ai (Co-founder & Non-Executive Director):Active (exists now)
; Research Funding (PI or named investigator):Novo Nordisk, Roche Diagnostics:Active (exists now)
; Consultant:Siemens Healthcare, Us2.ai:Active (exists now)
; Consultant:ReCor Medical, Roche Diagnostics, Sanofi:Active (exists now)
; Consultant:Prosciento Inc, Quidel Corporation, Radcliffe Group:Active (exists now)
; Consultant:Medscape, Merck, Novartis, Novo Nordisk:Active (exists now)
; Consultant:Ionis Pharmaceutical, Janssen R&D LLC:Active (exists now)
; Consultant:Impulse Dynamics, Intellia Therapeutics:Active (exists now)
; Consultant:CPC Clinical Research, Eli Lilly:Active (exists now)
; Consultant:Boston Scientific, Bristol Myers Squibb:Active (exists now)
; Consultant:Biopeutics, Boehringer Ingelheim:Active (exists now)
; Consultant:Astra Zeneca, Bayer:Active (exists now)
; Consultant:AnaCardio, Applied Therapeutics:Active (exists now)
| Mikhail Kosiborod: DO have relevant financial relationships
;
Consultant:35Pharma, Imbria Pharmaceuticals:Active (exists now)
; Consultant:Esperion Therapeutics, Structure Therapeutics:Active (exists now)
; Consultant:Eli Lilly, scPharmaceuticals:Active (exists now)
; Consultant:Dexcom, Sanofi, Youngene Therapeutics:Active (exists now)
; Consultant:Cytokinetics, Regeneron:Active (exists now)
; Consultant:Boehringer Ingelheim:Active (exists now)
; Research Funding (PI or named investigator):Boehringer Ingelheim:Active (exists now)
; Consultant:Bayer, Pharmacosmos:Active (exists now)
; Other (please indicate in the box next to the company name):AstraZeneca, Vifor Pharma - data analytic center fees:Active (exists now)
; Consultant:AstraZeneca, Pfizer, Vifor Pharma:Active (exists now)
; Research Funding (PI or named investigator):AstraZeneca, Pfizer:Active (exists now)
; Consultant:Arrowhead Pharmaceuticals:Active (exists now)
; Consultant:Applied Therapeutics:Active (exists now)
; Consultant:Amgen, NovoNordisk:Active (exists now)
; Consultant:Alnylam, Merck (Diabetes and Cardiovascular):Active (exists now)
| Silvio Inzucchi: No Answer
| Felipe Martinez: DO have relevant financial relationships
;
Researcher:AstraZeneca:Active (exists now)
; Researcher:Bayer:Active (exists now)
Meeting Info:
Session Info:
More abstracts on this topic:
A Case of Concomitant Wild-Type Transthyretin and Systemic Light Chain Amyloidosis Involving Separate Organs
Chiu Leonard, Afrough Aimaz, Nadeem Urooba, Jebakumar Deborah, Grodin Justin
Ambulatory Inotropic Support in Patients with Stage D Heart Failure Does Not Increase ICD TherapiesSunthankar Kathryn, Pfaff Jamie, Farber-eger Eric, Richardson Travis, Wells Quinn, Ooi Henry, Skelton Melanie, Stevenson Lynne, Pedrotty Dawn
More abstracts from these authors:
Lipid Profiles and Prognosis in Heart Failure: A Participant-Level Pooled Analysis of the PARADIGM-HF and PARAGON-HF Trials
Siqueira Sara, Jhund Pardeep, Mcmurray John, Vaduganathan Muthiah, Solomon Scott, Pabon Maria, Claggett Brian, Packer Milton, Lam Carolyn, Rouleau Jean, Zile Michael, Lefkowitz Marty, Desai Akshay
Race does not influence the efficacy and safety of mineralocorticoid-receptor antagonists in heart failure: An individual-participant data meta-analysis of 4 trialsButt Jawad, Pitt Bertram, Senni Michele, Shah Sanjiv, Zannad Faiez, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Talebi Atefeh, Vardeny Orly, Claggett Brian, Vaduganathan Muthiah, Desai Akshay, Lam Carolyn
You have to be authorized to contact abstract author. Please, Login
Not Available