Final ID: Mo2187
Systolic Blood Pressure in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: A Pooled Participant-Level Analysis of 4 Large-Scale Trials
Hypertension is common in patients with heart failure with preserved ejection fraction (HFpEF). Treatment of systolic blood pressure (SBP) to a target <130 mmHg in this population is often recommended based on general ACC/AHA and ESC hypertension guidelines but limited data are available to substantiate this recommendation in patients with HF. We aimed to explore the impact of baseline SBP on cardiovascular (CV) outcomes in a pooled dataset of 4 large, well-characterized cohorts of patients with HF with mildly reduced EF (mrEF) or HFpEF.
Methods
I-PRESERVE, TOPCAT, PARAGON-HF and DELIVER were global, multicenter, randomized clinical trials testing irbesartan, spironolactone, sacubitril/valsartan and dapagliflozin respectively, against either a placebo or an active control (valsartan, in PARAGON-HF), in patients with HF and a LVEF >40% (in DELIVER) or ≥45% (in all other 3 trials). The continuous relationship between baseline SBP and the incidence rates (IR) of 1st HF hospitalization (HFH) or CV death was analyzed with restricted cubic splines and adjusted for known risk factors of CV disease.
Results
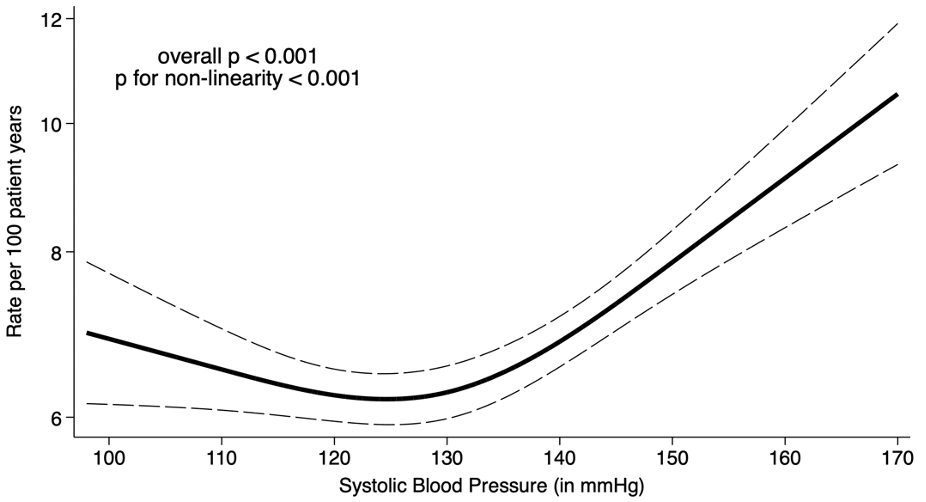
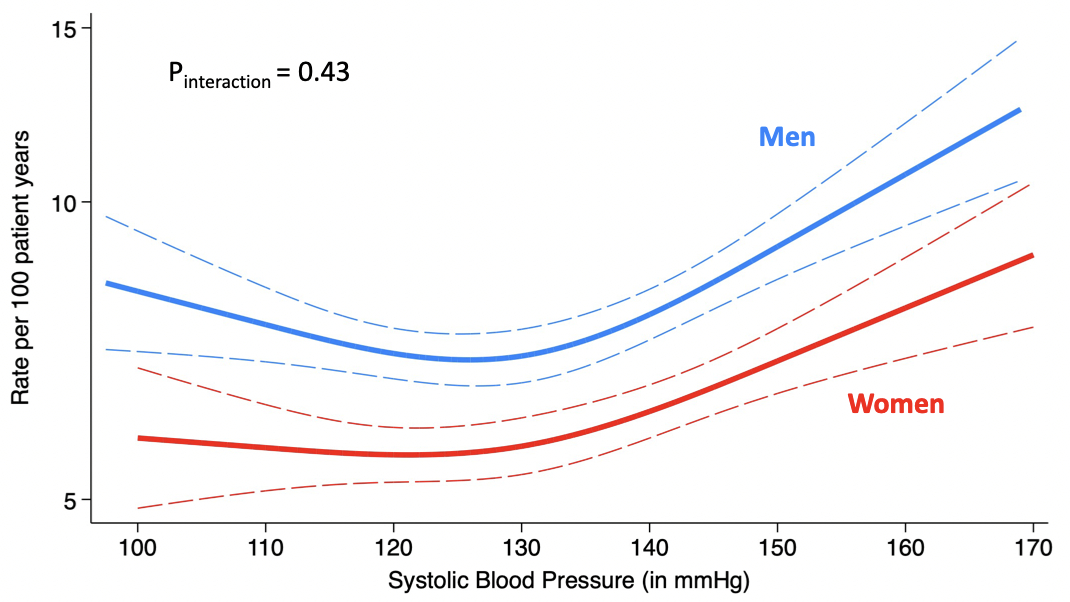
A total of 18,627 patients (mean age 71±9 years, 49% men, mean SBP 131±15 mmHg) were included. Over a median follow-up of 2.9 years, 3,852 patients experienced a first HFH or CV death (7.4 events per 100py). The relationship between the IR of 1st HFH or CV death and SBP appeared to be nonlinear, J-shaped (Figure 1), with a nadir of IR observed for SBP values between 120 and 130 mmHg, and an increase for SBP values above 130 mmHg. Above 130 mmHg, each 5 mmHg-increase in SBP was associated with a significantly higher risk of the primary outcome (adjusted HR: 1.07; 95% CI: 1.05-1.10). When stratifying the study population by sex, men had a higher risk for 1st HFH or CV death across the spectrum of SBP. The J-shaped relationship remained consistent in both men and women (Pinteraction=0.43; Figure 2).
Conclusions
Our analysis of a large pooled dataset from 4 clinical trials including over 18,600 patients with HFmrEF or pEF suggests a graded relationship between SBP and CV risk, with risk nadir at SBP between 120 to 130 mmHg, without evidence of difference according to sex.
- Lu, Henri ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Pfeffer, Marc ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Pitt, Bertram ( University of Michigan School , Ann Arbor , Michigan , United States )
- Zannad, Faiez ( INSERM-CIC , Vandoeuvre Les Nancy , France )
- Zile, Michael ( MEDICAL UNIV OF SOUTH CAROLINA , Charleston , South Carolina , United States )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Kondo, Toru ( Nagoya University , Nagoya , Japan )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Neuen, Brendon ( George Institute for Global Health , Newtown , New South Wales , Australia )
- Beldhuis, Iris ( UMCG , Groningen , Netherlands )
- Jhund, Pardeep ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Mc Causland, Finnian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Anand, Inder ( VA SAN DIEGO HEATHCARE SYSTEM , La Jolla , California , United States )
Meeting Info:
Session Info:
Frontiers in HFpEF Part 2: On-Treatment Effects, Predictors, and More!
Monday, 11/18/2024 , 01:30PM - 02:30PM
Abstract Poster Session
More abstracts on this topic:
A Longitudinal 20-year Analysis Indicates Acceleration of Cardiometabolic Comorbidities on Dementia Risk
Lihua Huang, Danish Muhammad, Auyeung Tw, Jenny Lee, Kwok Timothy, Abrigo Jill, Wei Yingying, Lo Cecilia, Fung Erik
A Key Role of Proximal Tubule Renin-Angiotensin System in The Kidney in The Development of Kidney Ischemia and Reperfusion InjuryLi Xiao, Hassan Rumana, Katsurada Akemi, Sato Ryosuke, Zhuo Jia
More abstracts from these authors:
Lu Henri, Pfeffer Marc, Pitt Bertram, Zannad Faiez, Zile Michael, Mcmurray John, Solomon Scott, Desai Akshay, Kondo Toru, Claggett Brian, Vaduganathan Muthiah, Neuen Brendon, Beldhuis Iris, Jhund Pardeep, Mc Causland Finnian, Anand Inder
Mode of Death in Heart Failure with Preserved Ejection across the Kidney Function Spectrum: Pooled Individual-Patient Level Analysis of 5 TrialsBart Nicole, Pfeffer Marc, Pitt Bertram, Zannad Faiez, Zile Michael, Mcmurray John, Solomon Scott, Cunningham Jonathan, Ariss Robert, Claggett Brian, Vaduganathan Muthiah, Neuen Brendon, Beldhuis Iris, Desai Akshay, Jhund Pardeep, Mc Causland Finnian