Final ID: VD8
Canonical And Noncanonical Heat Shock Factor 1 Signaling In Smooth Muscle Cells Promote Atherosclerotic Plaque Burden
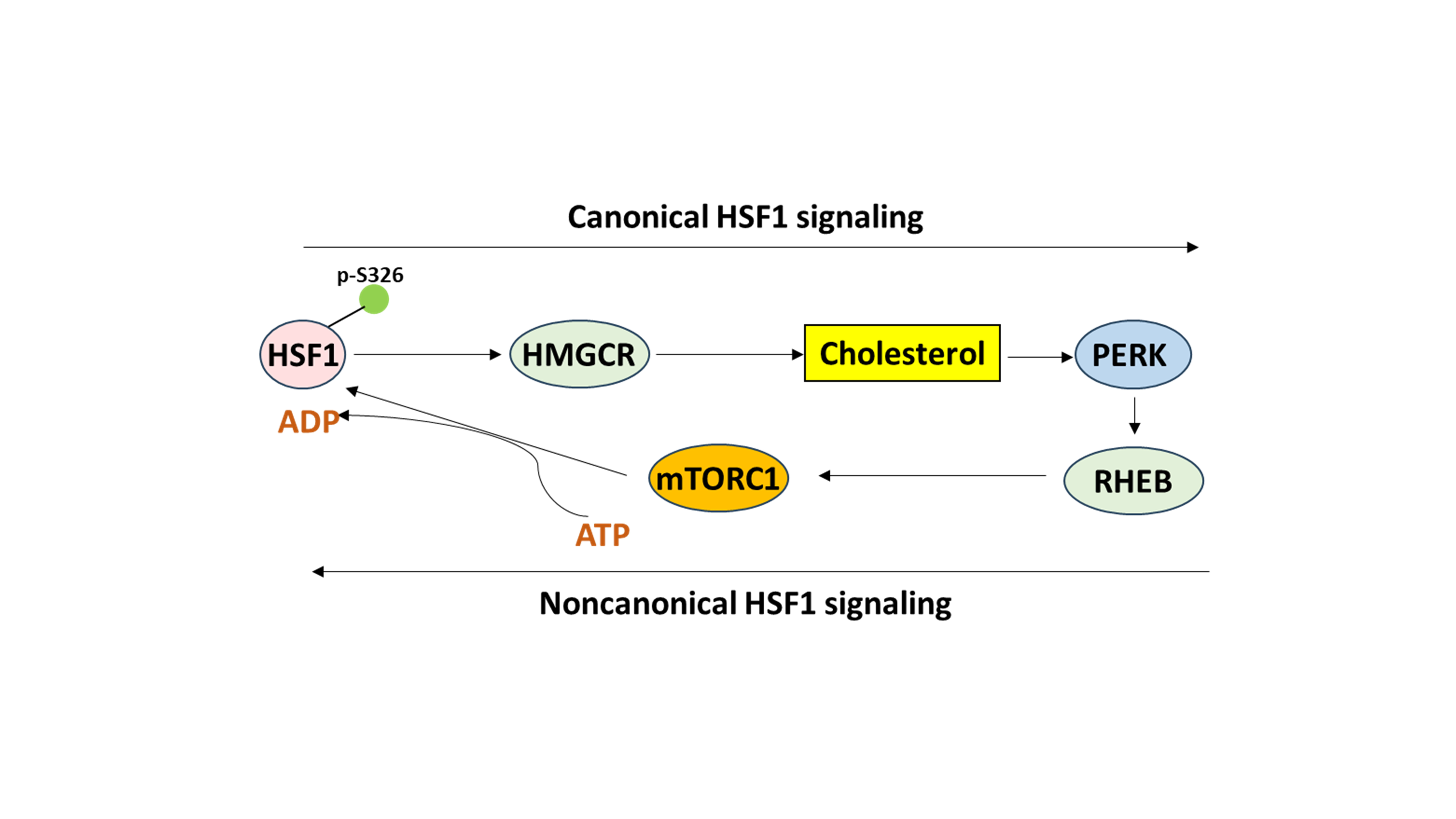
Abstract Body (Do not enter title and authors here): Smooth muscle cells (SMCs) in atherosclerotic plaques undergo complex phenotypic modulation, characterized by migration from the aortic medial layer to the intima, proliferation, de-differentiation, and variable increases in molecular markers characteristic of macrophages, fibroblasts, osteogenic cells and mesenchymal stem cells. This transition is driven in part by cholesterol entering the endoplasmic reticulum (ER) and activating ER stress pathways. The importance of ER stress-driven PERK signaling in atherosclerosis is underscored by the observation that SMC-specific deletion of Perk reduces lesion size by ~70% in hypercholesterolemic (HC) mice. HC mice harboring either an SM alpha-actin (ACTA2) missense variant or SMC-specific deletion of the centrosomal scaffolding protein pericentrin have augmented plaque burden compared to their HC wildtype (WT) counterparts, despite having comparable serum lipid levels. We went on to demonstrate that both genetic alterations increase SMC cytosolic stress and activation of canonical heat shock factor 1 (HSF1) signaling, which in turn increases HMG-CoA reductase (HMGCR) activity and cholesterol biosynthesis, ER stress, PERK signaling, SMC phenotypic modulation and finally, an augmented plaque burden. SMC-specific loss of Hsf1 in HC mice reduces plaque burden by ~67% in the whole aorta of HC mice (induced by a single injection of AAV-PCSK9DY), followed by a high fat diet for 12 weeks, compared to similarly treated WT mice (N=12, males only, p=0.0007, student’s t-test, followed by Welch’s correction). Interestingly, SMCs in culture exposed to high levels of exogenous cholesterol activate both PERK and HSF1, and blocking PERK signaling prevents HSF1 activation, suggesting the existence of “noncanonical” HSF1 activation. Mechanistically, PERK activates the mechanistic target of rapamycin complex 1 (mTORC1), which activates HSF1 by phosphorylation. These data suggest distinct roles of canonical (HSF1-HMGCR-PERK) and noncanonical (PERK-mTORC1-HSF1) HSF1 signaling in SMCs that contribute to atherosclerotic plaque burden.
More abstracts on this topic:
A major uremic toxin indoxyl sulfate impairs macrophage efferocytosis and accelerates atherogenesis: a potential mechanism for cardiovascular risk in chronic kidney disease
Jha Prabhash, Kasai Taku, Vromman Amelie, Holden Rachel, Libby Peter, Tabas Ira, Singh Sasha, Aikawa Elena, Aikawa Masanori, Lupieri Adrien, Sonawane Abhijeet, Le Thanh-dat, Becker-greene Dakota, Chelvanambi Sarvesh, Turner Mandy, Nakamura Yuto, Passos Livia
Acute Electronic Cigarette Exposure Induces Heat Shock Response and Protein Aggregation in Vascular Smooth Muscle CellsDamiani Isabella, Huiting Wouter, Qin Guyu, Basu Sugandha, Easwaran Meena, Direnzo Elizabeth, Jarosz Daniel, Kim Juyong