Final ID: Mo3130
Treatment Satisfaction With an “Inclisiran First” Implementation Strategy Versus Usual Care in Patients With Atherosclerotic Cardiovascular Disease
Abstract Body (Do not enter title and authors here): Background: Despite the availability of effective lipid-lowering therapies (LLTs), most patients (pts) with ASCVD do not meet guideline-recommended LDL-C goals. In VICTORION-INITIATE (NCT04929249), an “inclisiran first” (IF) strategy of adding inclisiran immediately on failure to achieve LDL-C <70 mg/dL, despite maximally tolerated statin therapy, in pts with ASCVD led to significantly higher LDL-C goal attainment vs usual care (UC) at Day 330.
Objective: To evaluate change from baseline to Day 330 in pt-reported treatment satisfaction with an IF implementation strategy vs UC in pts with ASCVD enrolled in VICTORION-INITIATE.
Methods: VICTORION-INITIATE, a Phase 3b, open-label, prospective trial, was conducted at 45 representative clinical sites across 20 US states. Pts randomized 1:1 (stratified by insurance status) received inclisiran 284 mg (300 mg inclisiran sodium) at Days 0, 90, and 270 plus UC or UC alone (lipid management directed by treating physicians). We evaluated change from baseline to Day 330 in pt-reported satisfaction with IF vs UC using the validated 11-question Treatment Satisfaction Questionnaire for Medication (TSQM)-II comprising 4 domains (effectiveness, side effects, convenience, and global satisfaction) scored from 0–100. As a post-hoc analysis of a pre-specified exploratory endpoint, we analyzed the difference in least-squares (LS) mean change in TSQM-II domain scores for IF vs UC using ANCOVA with baseline scores as a covariate.
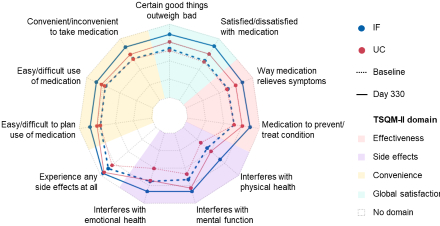
Results: We randomized 450 patients: median age 67 years, 30.9% female, 25.8% with a history of statin intolerance. From baseline to Day 330, difference in LS mean change (95% confidence interval) in TSQM-II scores for IF (n=139) vs UC (n=105) was significant (p<0.001) for effectiveness (10.4 [5.4, 15.5]), convenience (10.5 [5.8, 15.2]), and global satisfaction (12.1 [7.2, 16.9]). Statistical comparison was not applicable for the side effects domain at Day 330 due to the small sample size (IF: n=6, UC: n=7). On Day 330, mean scores for IF exceeded UC for each TSQM-II question (Figure).
Conclusions: An IF implementation strategy resulted in significantly greater change from baseline to Day 330 across multiple domains of pt-reported treatment satisfaction vs UC.
Objective: To evaluate change from baseline to Day 330 in pt-reported treatment satisfaction with an IF implementation strategy vs UC in pts with ASCVD enrolled in VICTORION-INITIATE.
Methods: VICTORION-INITIATE, a Phase 3b, open-label, prospective trial, was conducted at 45 representative clinical sites across 20 US states. Pts randomized 1:1 (stratified by insurance status) received inclisiran 284 mg (300 mg inclisiran sodium) at Days 0, 90, and 270 plus UC or UC alone (lipid management directed by treating physicians). We evaluated change from baseline to Day 330 in pt-reported satisfaction with IF vs UC using the validated 11-question Treatment Satisfaction Questionnaire for Medication (TSQM)-II comprising 4 domains (effectiveness, side effects, convenience, and global satisfaction) scored from 0–100. As a post-hoc analysis of a pre-specified exploratory endpoint, we analyzed the difference in least-squares (LS) mean change in TSQM-II domain scores for IF vs UC using ANCOVA with baseline scores as a covariate.
Results: We randomized 450 patients: median age 67 years, 30.9% female, 25.8% with a history of statin intolerance. From baseline to Day 330, difference in LS mean change (95% confidence interval) in TSQM-II scores for IF (n=139) vs UC (n=105) was significant (p<0.001) for effectiveness (10.4 [5.4, 15.5]), convenience (10.5 [5.8, 15.2]), and global satisfaction (12.1 [7.2, 16.9]). Statistical comparison was not applicable for the side effects domain at Day 330 due to the small sample size (IF: n=6, UC: n=7). On Day 330, mean scores for IF exceeded UC for each TSQM-II question (Figure).
Conclusions: An IF implementation strategy resulted in significantly greater change from baseline to Day 330 across multiple domains of pt-reported treatment satisfaction vs UC.
More abstracts on this topic:
A 3-Year, Pre-Trial, Real-world Data Analysis of Patients Enrolled in VICTORION-INITIATE: Insights Using Tokenization
Rodriguez Fatima, Cosmatos Irene, Desai Nihar, Wright R, Ross Elsie, Ali Yousuf, Kumar Biswajit, Han Guangyang, Cai Beilei, Abbas Cheryl, Ryan Amy
A 10-year longitudinal cohort study of lipid variability, cognitive decline, and dementia in 9846 community-dwelling older adultsZhou Zhen, Moran Chris, Murray Anne, Zoungas Sophia, Nelson Mark, Talic Stella, Wolfe Rory, Ryan Joanne