Final ID: MDP128
Beta blockers and calcium channel blockers in pre-symptomatic patients with hypertrophic cardiomyopathy: prevalence, discontinuation, and effectiveness
Beta-blockers (BB) and non-dihydropyridine calcium channel blockers (CCB) are first-line therapies for hypertrophic cardiomyopathy (HCM) used both upon symptom onset and for pre-symptomatic patients. However, there is limited evidence supporting their efficacy, particularly from randomized controlled trials.
Aims
In this study we assessed the prevalence, discontinuation rates, and effectiveness of BB/CCB for NYHA class I HCM patients.
Methods
Utilizing data from a real world cohort spanning 4 centers, we evaluated BB/CCB effectiveness in NYHA class I patients with HCM. The effectiveness analysis for a composite endpoint (NYHA class worsening and cardiovascular-related hospitalization) was performed independently at each center and an aggregated analysis was performed. NT-proBNP level analysis was performed where longitudinal data was available. Patients initiating therapy during follow-up were compared to untreated controls, adjusting for confounders (duration since HCM diagnosis, left atrial volume index (LAVi), E/e' lateral ratio, medical history, NT-ProBNP levels) using inverse probability weighting and Stürmer trimming. The association between treatment and the risk of composite endpoint was assessed through Cox regression, while mixed models for repeated measures were used for NT-proBNP levels.
Results
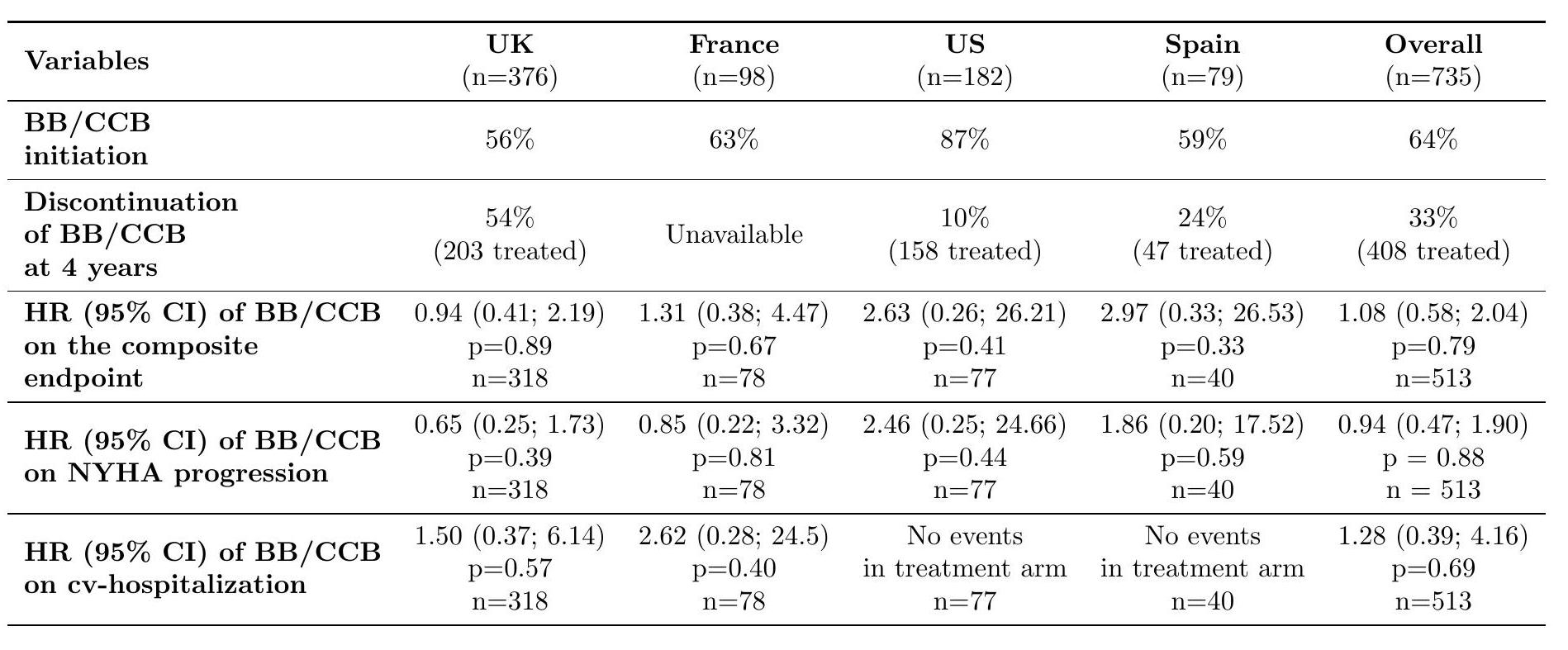
Among the 735 patients, 64% initiated BB/CCB therapy with a 33% discontinuation rate at 4 years. A total of 222 patients with extreme propensity scores were excluded. As shown in Table 1., no benefit from BB/CCB in reducing the composite endpoint risk (HR=1.08, p=0.79) was observed. This was consistent across the different components of the endpoint. Additionally, no benefit in reducing NT-proBNP levels after 1 year was detected (Least Means Square difference=0.02, p=0.79).
Conclusion
Initiation of BB/CCB for NYHA I HCM patients did not appear to reduce the risk of progression measured by the functional capacity, CV hospitalization and NT-proBNP. Despite the inherent limitation of observational studies, these findings together with the high discontinuation rate of the therapy, underscore the need for further research to validate optimal treatment strategies for pre-symptomatic HCM patients.
- Bradlow, William ( University Hospitals Birmingham NHS Foundation Trust , Birmingham , United Kingdom )
- Sehnert, Amy ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Bastien, Arnaud ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Blizard, Perry ( University Hospitals Birmingham NHS Foundation Trust , Birmingham , United Kingdom )
- Ripoll-vera, Tomas ( SON LLATZER UNIVERSITY HOSPITAL , Palma Mallorca , Spain )
- Pericas, Pau ( SON LLATZER UNIVERSITY HOSPITAL , Palma Mallorca , Spain )
- Fudim, Marat ( Duke University Heart Center , Durham , North Carolina , United States )
- Balu, Suresh ( Duke Institute for Health Innovation , Durham , North Carolina , United States )
- Hintze, Bradley ( Duke Institute for Health Innovation , Durham , North Carolina , United States )
- Salah, Husam ( Duke University Heart Center , Durham , North Carolina , United States )
- Patel, Manesh ( Duke University Heart Center , Durham , North Carolina , United States )
- Elgui, Kevin ( Owkin , Paris , France )
- Foucher, Aurelie ( AP-HP - Centre de référence des maladies cardiaques héréditaires ou rares , Paris , France )
- Charron, Philippe ( AP-HP - Centre de référence des maladies cardiaques héréditaires ou rares , Paris , France )
- Klopfenstein, Quentin ( Owkin , Paris , France )
- Balazard, Felix ( Owkin , Paris , France )
- Trichelair, Paul ( Owkin , Paris , France )
- Touzot, Maxime ( Owkin , Paris , France )
- Micsinai Balan, Mariann ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Van Haelst, Paul ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Sandler, Belinda ( Bristol Myers Squibb , Princeton , New Jersey , United States )
Meeting Info:
Session Info:
Bulking Up: The Latest in Hypertrophic Cardiomyopathy
Saturday, 11/16/2024 , 02:50PM - 04:05PM
Moderated Digital Poster Session
More abstracts on this topic:
Kulkarni Priyanka, Molk Matthew, Wright Shannon, Calvet Marianna, Pepine Carl, Schmidt Stephan, Vouri Scott, Morris Earl, Smith Steven, Ndai Asinamai, Smith Kayla, Keshwani Shailina, Choi Jaeyoung, Luvera Michael, Hunter Julia, Galvan Rebecca, Beachy Tanner
AI-enhanced Electrocardiographic Evaluation of Left Ventricular Ejection Fraction and Outflow Tract Gradient in Hypertrophic CardiomyopathySangha Veer, Aminorroaya Arya, Dhingra Lovedeep, Pedroso Aline, Oikonomou Evangelos, Khera Rohan
More abstracts from these authors:
Fudim Marat, Weerts Jerremy, Patel Manesh, Balu Suresh, Hintze Bradley, Torres Francisco, Micsinai Balan Mariann, Rigolli Marzia, Kessler Paul, Touzot Maxime, Lund Lars, Van Empel Vanessa, Pradhan Aruna, Butler Javed, Zehnder Tobias, Sauty Benoit, Esposito Christian, Balazard Félix, Mayer Imke, Hallal Mohammad, Loiseau Nicolas
Investigating the natural history and risk factors underlying deterioration in early-stage hypertrophic cardiomyopathy: findings from SOLENOIDBradlow William, Guichard Ninon, Jaeger Alexandre, Micsinai Balan Mariann, Simon Antoine, Sehnert Amy, Touzot Maxime, Bastien Arnaud, Charron Philippe, Elgui Kevin, Ducret Valerie, Trichelair Paul, Chevalier Philippe, Ripoll Tomas, Fudim Marat, Patel Manesh, Deleforge Aurelie