Final ID: EPI10
A Randomized Placebo-Controlled Trial of Pitavastatin Calcium to Treat Combined Dyslipidemia of Obesity in Adolescents – The Pediatric Heart Network Dyslipidemia of Obesity Intervention in Teens (DO IT!) Trial
Methods: A 2-year randomized trial of pitavastatin calcium 4 mg/day versus placebo for participants ages 10-19 years with body mass index (BMI) >85th %ile and CDO defined as non-HDL-C >120 mg/dL and either low HDL-C or high triglyceride (TG):HDL-C ratio was performed. The primary outcome was change in carotid-femoral pulse wave velocity (PWV) assessed at baseline, 6, 12, 18, and 24 months. Secondary outcomes included safety and lipid measures.
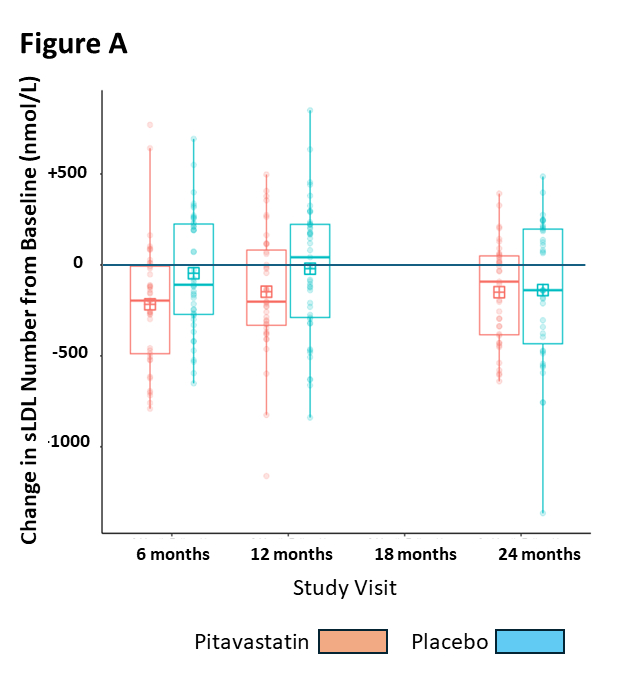
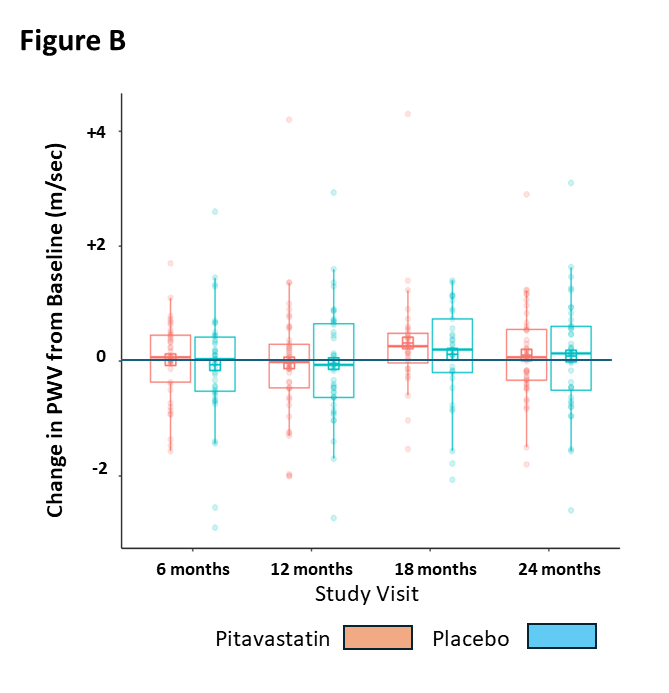
Results: Across 18 sites, 59 participants were randomized to pitavastatin and 60 to placebo. Demographic (mean age 13.5+1.2 yrs; 55% males), anthropomorphic (BMI %ile 98+3; waist/height ratio 0.66+0.08), lipid (non-HDL-C 167+29 mg/dL, LDL-C 127+25 mg/dL, HDL-C 37+7 mg/dL, TG/HDL ratio 6.4+4.7, sLDL number 866+419 nmol/L), vascular (PWV 5.0+0.7 m/sec) and safety measures were similar at baseline between groups. There were 28 early terminations (11 pitavastatin, 17 placebo). Significant reductions with pitavastatin versus placebo were noted in non-HDL-C (median -25% vs +3% at 6 months, p<0.001), LDL-C (-29% vs +7%, p<0.001), and apolipoprotein B (-26% vs 0%, p<0.001). An early reduction relative to placebo in sLDL particle number was not sustained (FIGURE A), and there were no significant changes or trends for PWV (FIGURE B) in either group. There was one serious adverse event (placebo), and no significant differences in liver enzymes, muscle toxicity, glucose homeostasis, or linear growth, including adiposity which did not change over the course of the study.
Conclusions: Over 2 years, treatment of CDO in adolescents with pitavastatin calcium resulted in significant improvement in dyslipidemia with no safety concerns. No change in PWV in either group may be due to lower-than-expected baseline PWV, insufficient magnitude of impact on CDO, or lack of change in adiposity.
- De Ferranti, Sarah ( Children's Hospital Boston , Boston , Massachusetts , United States )
- Cartoski, Mark ( Nemours/Alfred I DuPont Hospital for Children , Wilmington , Delaware , United States )
- Brothers, Julie ( Children's Hospital of Philadelphia , Philadelphia , Pennsylvania , United States )
- San Giovanni, Christine ( Medical University of South Carolina , Charleston , South Carolina , United States )
- Zachariah, Justin ( Texas Children's Hospital , Houston , Texas , United States )
- Pena, Sandra ( Texas Children's Hospital , Houston , Texas , United States )
- Mahle, William ( Children's Health Care of Atlanta , Atlanta , Georgia , United States )
- Peterson, Amy ( University of Wisconsin School of Medicine and Public Health , Madison , Wisconsin , United States )
- Magge, Sheela ( Johns Hopkins University School of Medicine , Baltimore , Maryland , United States )
- Raghuveer, Geetha ( Children's Mercy Hospital , Kansas City , Missouri , United States )
- Sharma, Binu ( Carelon Research , Newton , Massachusetts , United States )
- Arslanian, Md, Silva ( Children's Hospital of Pittsburgh , Pittsurgh , Pennsylvania , United States )
- Kazlova, Valiantsina ( Carelon Research , Newton , Massachusetts , United States )
- Sponseller, Craig ( Kowa Pharmaceuticals America , Montgomery , Alabama , United States )
- Freemon, Dandrea ( National Heart, Lung and Blood Institute , Bethesda , Maryland , United States )
- Stylianou, Mario ( National Heart, Lung and Blood Institute , Bethesda , Maryland , United States )
- Mccrindle, Brian ( The Hospital for Sick Children , Toronto , Ontario , Canada )
- Mietus-snyder, Michele ( Children's National Hospital , Washington , District of Columbia , United States )
- Urbina, Elaine ( Cincinnati Children's Hospital Medical Center , Cincinnati , Ohio , United States )
- Ware, Adam ( Primary Children's Hospital , Salt Lake City , Utah , United States )
- Teng, Jessica ( Carelon Research , Newton , Massachusetts , United States )
- Trachtenberg, Felicia ( Carelon Research , Newton , Massachusetts , United States )
- Russell, Mark ( CS Mott Children's Hospital , Ann Arbor , Michigan , United States )
- Shah, Amy ( Cincinnati Children's Hospital Medical Center , Cincinnati , Ohio , United States )
Meeting Info:
Session Info:
Best of AHA Specialty Conferences: EPI/Lifestyle 2024
Monday, 11/18/2024 , 10:30AM - 11:30AM
Best of Specialty Conferences
More abstracts on this topic:
Ajibewa Tiwaloluwa, Master Lindsay, Booker Robert, Wong Mandy, Reichenberger David, Mathew Gina, Buxton Orfeu, Chang Anne-marie, Hale Lauren
Atorvastatin and Pulse Wave Velocity in Anthracycline-based ChemotherapyJuhasz Vencel, Han Yuchi, Ky Bonnie, Kwong Raymond, Januzzi James, Asnani Aarti, Mousavi Negareh, Redd Robert, Jerosch-herold Michael, Scherrer-crosbie Marielle, Neilan Tomas, Drobni Zsofia, Quinaglia Thiago, Gilman Hannah, Brendel Jan, Suero-abreu Giselle, Ghamari Azin, Heemelaar Julius, Neuberg Donna
More abstracts from these authors:
Peterson Amy, Ware Adam
Gathering institutional support for pediatric preventive cardiology program developmentPeterson Amy, Ryan Heather