Scientific Sessions 2024
/
Genomics and Informatics
/
Integrating genomics and proteomics to identify causal proteins and biologic pathways for incident atrial fibrillation in chronic kidney disease
Final ID: MDP789
Integrating genomics and proteomics to identify causal proteins and biologic pathways for incident atrial fibrillation in chronic kidney disease
Abstract Body (Do not enter title and authors here):
Introduction/ Background:
Individuals with chronic kidney disease (CKD) are at an increased risk for developing atrial fibrillation (AF).
Research Objective:
By integrating genomics and proteomics, we sought to identify proteins and biologic pathways causally linked to the development of AF in the CKD population.
Methods / Approach:
We measured 4638 unique plasma proteins using SomaScan v.4.0 in participants (ppts) with CKD and no prior AF from 2 cohorts: Chronic Renal Insufficiency Cohort (CRIC, n=2654) and Atherosclerosis Risk in Communities Cohort (ARIC, n=1326). Associations of individual proteins with incident AF were analyzed using Cox regression after adjustment for demographics, comorbidities, and kidney function. Among proteins independently associated with incident AF in both cohorts, we tested for causality using two-sample Mendelian Randomization (MR). Previously published genome wide association studies (GWAS) were used to identify both protein quantitative loci (cis-pQTL) and summary statistics for incident AF. We then used Ingenuity Pathway Analysis to compare proteins and pathways associated with both incident AF and an increase in left atrial (LA) size across serial echocardiograms in CRIC.
Results / Data:
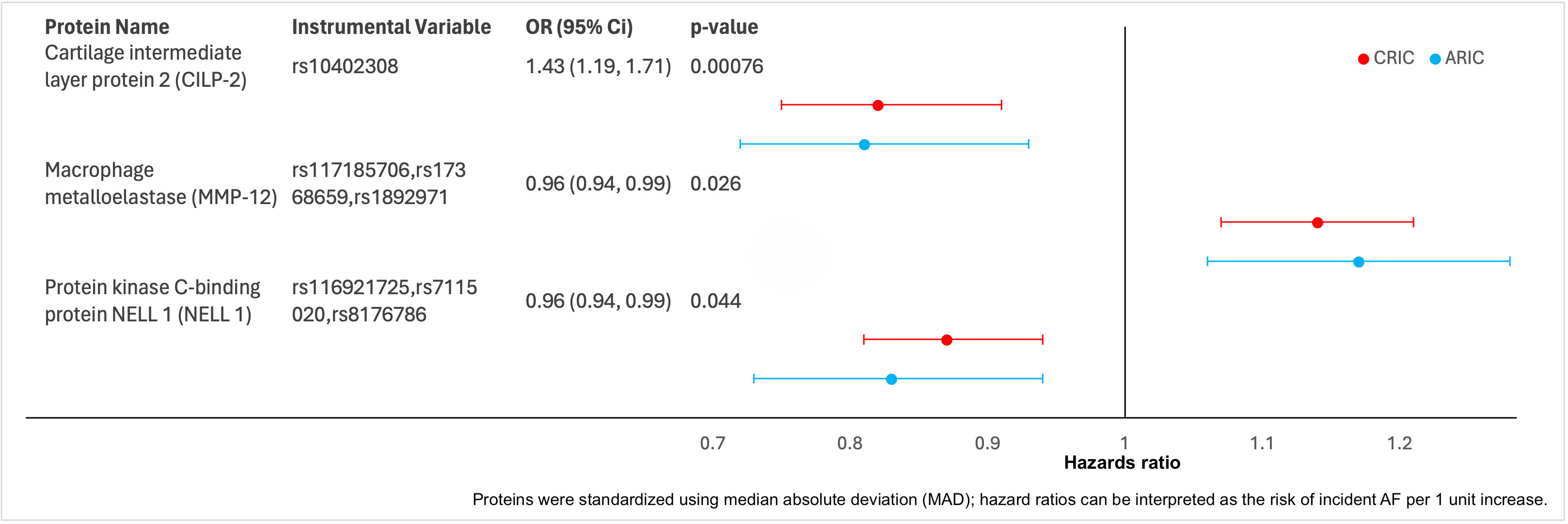
Over the 5-year analytic period, incident AF occurred in 150 ppts from CRIC and 140 ppts from ARIC. Eight proteins were independently associated with AF in both cohorts: NT-proBNP, SVEP1, TAGLN, TFF3, WAP4, MMP-12, CILP2 and NELL-1. Of these, 3 were significant in MR analysis (Figure 1): CILP2 and MMP-12 maintain structural integrity of the extracellular matrix in the heart and modulate the fibrotic response; NELL-1 is responsible for cell signaling in kidney disease and cardiovascular development. In addition, leading biologic pathways implicated in both development of AF and increase in LA size included systemic fibrosis (p=1.7x10-4 for AF and 2.3 x10-4 for LA); regulation of insulin-like growth factor (p=2.3 x10-4 for AF and p=4.3 x10-7 for LA) and extracellular matrix degradation (p=5.5 x10-4 for AF and p=4.2 x10-4 for LA).
Conclusions:
Trans-omics analyses identified 3 proteins causally associated with developing AF in the CKD population. Also, several pathways involved with cardiac remodeling and fibrosis are common to the development of AF and LA remodeling.
Introduction/ Background:
Individuals with chronic kidney disease (CKD) are at an increased risk for developing atrial fibrillation (AF).
Research Objective:
By integrating genomics and proteomics, we sought to identify proteins and biologic pathways causally linked to the development of AF in the CKD population.
Methods / Approach:
We measured 4638 unique plasma proteins using SomaScan v.4.0 in participants (ppts) with CKD and no prior AF from 2 cohorts: Chronic Renal Insufficiency Cohort (CRIC, n=2654) and Atherosclerosis Risk in Communities Cohort (ARIC, n=1326). Associations of individual proteins with incident AF were analyzed using Cox regression after adjustment for demographics, comorbidities, and kidney function. Among proteins independently associated with incident AF in both cohorts, we tested for causality using two-sample Mendelian Randomization (MR). Previously published genome wide association studies (GWAS) were used to identify both protein quantitative loci (cis-pQTL) and summary statistics for incident AF. We then used Ingenuity Pathway Analysis to compare proteins and pathways associated with both incident AF and an increase in left atrial (LA) size across serial echocardiograms in CRIC.
Results / Data:
Over the 5-year analytic period, incident AF occurred in 150 ppts from CRIC and 140 ppts from ARIC. Eight proteins were independently associated with AF in both cohorts: NT-proBNP, SVEP1, TAGLN, TFF3, WAP4, MMP-12, CILP2 and NELL-1. Of these, 3 were significant in MR analysis (Figure 1): CILP2 and MMP-12 maintain structural integrity of the extracellular matrix in the heart and modulate the fibrotic response; NELL-1 is responsible for cell signaling in kidney disease and cardiovascular development. In addition, leading biologic pathways implicated in both development of AF and increase in LA size included systemic fibrosis (p=1.7x10-4 for AF and 2.3 x10-4 for LA); regulation of insulin-like growth factor (p=2.3 x10-4 for AF and p=4.3 x10-7 for LA) and extracellular matrix degradation (p=5.5 x10-4 for AF and p=4.2 x10-4 for LA).
Conclusions:
Trans-omics analyses identified 3 proteins causally associated with developing AF in the CKD population. Also, several pathways involved with cardiac remodeling and fibrosis are common to the development of AF and LA remodeling.
- Petzl, Adrian ( Hospital University Pennsylvania , Philadelphia , Pennsylvania , United States )
- Surapaneni, Aditya ( Johns Hopkins University , Baltimore , Maryland , United States )
- Coresh, Joe ( JOHNS HOPKINS SPH WELCH CENTER , Baltimore , Maryland , United States )
- Grams, Morgan ( NYU Grossman School of Medicine , New York , New York , United States )
- Feldman, Harold ( UPenn Perelman School of Medicine , Philadelphia , Pennsylvania , United States )
- Bansal, Nisha ( University of Washington , Seattle , Washington , United States )
- Marchlinski, Francis ( University of Pennsylvania , Philadelphia , Pennsylvania , United States )
- Go, Alan ( KAISER PERMANENTE NORTHERN CAL , Oakland , California , United States )
- Ganz, Peter ( Zuckerberg San Francisco General , Belvedere , California , United States )
- Deo, Rajat ( Hospital University Pennsylvania , Philadelphia , Pennsylvania , United States )
- Norby, Faye ( University of Minnesota , Minneapolis , Minnesota , United States )
- Ren, Yue ( University of Pennsylvania , Philadelphia , Pennsylvania , United States )
- Wang, Jianqiao ( Harvard T.H. Chan School of Public Health , Boston , Massachusetts , United States )
- Dubin, Ruth ( UT Southwestern Medical Center , Dallas , Texas , United States )
- Li, Hongzhe ( University of Pennsylvania , Philadelphia , Pennsylvania , United States )
- Segal, Mark ( UCSF , SAN CARLOS , California , United States )
- Chen, Lin ( UNIVERSITY OF MINNESOTA , Minneapolis , Minnesota , United States )
- Alonso, Alvaro ( Emory University , Atlanta , Georgia , United States )
Author Disclosures:
Adrian Petzl: DO NOT have relevant financial relationships
| Aditya Surapaneni: No Answer
| Joe Coresh: No Answer
| Morgan Grams: DO NOT have relevant financial relationships
| Harold Feldman: No Answer
| Nisha Bansal: No Answer
| Francis Marchlinski: No Answer
| Alan Go: DO have relevant financial relationships
;
Research Funding (PI or named investigator):Bristol Myers-Squibb:Active (exists now)
; Research Funding (PI or named investigator):Pfizer:Past (completed)
| Peter Ganz: No Answer
| Rajat Deo: DO NOT have relevant financial relationships
| Faye Norby: DO NOT have relevant financial relationships
| Yue Ren: DO NOT have relevant financial relationships
| Jianqiao Wang: No Answer
| Ruth Dubin: No Answer
| Hongzhe Li: No Answer
| Mark Segal: DO NOT have relevant financial relationships
| Lin Chen: DO NOT have relevant financial relationships
| Alvaro Alonso: DO NOT have relevant financial relationships
Meeting Info:
Session Info:
More abstracts on this topic:
A major uremic toxin indoxyl sulfate impairs macrophage efferocytosis and accelerates atherogenesis: a potential mechanism for cardiovascular risk in chronic kidney disease
A Large Animal Model of Persistent Atrial Fibrillation
Jha Prabhash, Kasai Taku, Vromman Amelie, Holden Rachel, Libby Peter, Tabas Ira, Singh Sasha, Aikawa Elena, Aikawa Masanori, Lupieri Adrien, Sonawane Abhijeet, Le Thanh-dat, Becker-greene Dakota, Chelvanambi Sarvesh, Turner Mandy, Nakamura Yuto, Passos Livia
A Large Animal Model of Persistent Atrial Fibrillation
Mostafizi Pouria, Goldman Steven, Moukabary Talal, Lefkowitz Eli, Ref Jacob, Daugherty Sherry, Grijalva Adrian, Cook Kyle Eric, Chinyere Ike, Lancaster Jordan, Koevary Jen
More abstracts from these authors:
Fitbit-Measured Physical Activity Is Inversely Associated With Incident Atrial Fibrillation Among All Of Us Participants
Barua Souptik, Upadhyay Dhairya, Surapaneni Aditya, Grams Morgan, Heffron Sean
An Echocardiogram-Based Risk Model to Predict Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) StudySun Daokun, Chen Lin, Chan Lap Sum, Norby Faye, Inciardi Riccardo, Soliman Elsayed, Alonso Alvaro, Solomon Scott, Shah Amil, Pan Wei
You have to be authorized to contact abstract author. Please, Login
Not Available