Final ID: MDP12
Effect of mild hepatic impairment on the pharmacokinetics of pelacarsen
Abstract Body (Do not enter title and authors here): Introduction: Pelacarsen, a hepatocyte-directed, N-acetyl galactosamine (GalNAc3)–conjugated antisense oligonucleotide, reduces plasma lipoprotein(a) levels by inhibiting apolipoprotein(a) translation. Pelacarsen uptake is mediated by GalNAc3 binding to the hepatocyte-specific asialoglycoprotein receptor.
Hypothesis: It is unknown whether hepatic impairment (HI) impacts pelacarsen uptake and systemic exposure.
Aim: This single-dose, open-label, parallel-group, Phase 1 study (NCT05026996) assessed the pharmacokinetics (PK), safety, and tolerability of a single 80 mg subcutaneous dose of pelacarsen in participants with mild HI compared to healthy controls (normal hepatic function).
Methods: Eight adults with prior liver cirrhosis and mild HI (Child-Pugh Class A) were matched for sex, age, and body weight with nine healthy controls. PK parameters (Cmax, AUC0-72, AUClast, and AUCinf) were determined using non-compartmental methods. Log-transformed PK parameters were analyzed using a statistical model with group and matching covariates as fixed effects. Least-square geometric means for each group and geometric mean ratios between participants with mild HI and healthy controls were extracted. Safety was also assessed.
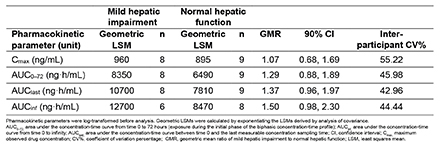
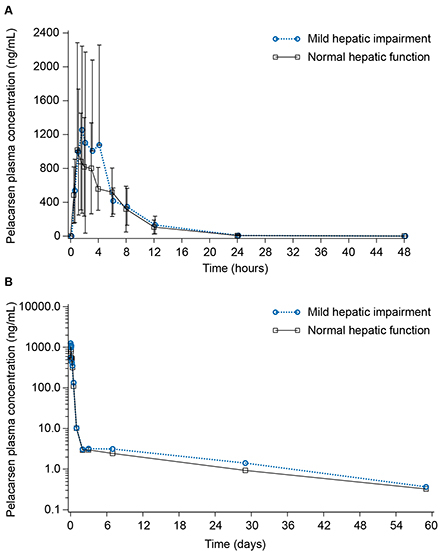
Results: Pelacarsen Cmax, AUClast, and AUCinf were, on average, 7%, 37%, and 50% higher, respectively, in participants with mild HI versus matched controls. All 90% confidence intervals around the HI versus healthy control geometric mean ratios included 1 (Table). The ranges of all PK parameters and estimated half-lives were similar between groups. In participants with mild HI, pelacarsen exposure approached the same level as controls after eight hours post-dose (Figure). No serious adverse events occurred.
Conclusion(s): In participants with mild HI, pelacarsen was well tolerated. Mild HI had no significant effect on pelacarsen Cmax. The non-statistically significant transient increase in AUC was within the exposure range tested in the first-in-human study.
Hypothesis: It is unknown whether hepatic impairment (HI) impacts pelacarsen uptake and systemic exposure.
Aim: This single-dose, open-label, parallel-group, Phase 1 study (NCT05026996) assessed the pharmacokinetics (PK), safety, and tolerability of a single 80 mg subcutaneous dose of pelacarsen in participants with mild HI compared to healthy controls (normal hepatic function).
Methods: Eight adults with prior liver cirrhosis and mild HI (Child-Pugh Class A) were matched for sex, age, and body weight with nine healthy controls. PK parameters (Cmax, AUC0-72, AUClast, and AUCinf) were determined using non-compartmental methods. Log-transformed PK parameters were analyzed using a statistical model with group and matching covariates as fixed effects. Least-square geometric means for each group and geometric mean ratios between participants with mild HI and healthy controls were extracted. Safety was also assessed.
Results: Pelacarsen Cmax, AUClast, and AUCinf were, on average, 7%, 37%, and 50% higher, respectively, in participants with mild HI versus matched controls. All 90% confidence intervals around the HI versus healthy control geometric mean ratios included 1 (Table). The ranges of all PK parameters and estimated half-lives were similar between groups. In participants with mild HI, pelacarsen exposure approached the same level as controls after eight hours post-dose (Figure). No serious adverse events occurred.
Conclusion(s): In participants with mild HI, pelacarsen was well tolerated. Mild HI had no significant effect on pelacarsen Cmax. The non-statistically significant transient increase in AUC was within the exposure range tested in the first-in-human study.
More abstracts on this topic:
A Selective Agonist of the Beta 2 Retinoic Acid Receptor Reduces Necrosis, Lipid Peroxidation, and Oxidative Stress in Doxorubicin-induced Cardiotoxicity
Varzideh Fahimeh, Jankauskas Stanislovas, Kansakar Urna, Tang Xiao-han, Gudas Lorraine, Levi Roberto, Gambardella Jessica, Santulli Gaetano
A novel Urocortin-2 analog COR-1167 corrects cardiac and renal dysfunction on top of Empagliflozin in a rat model of acute decompensated heart failureStephan Yohan, Corruble Clement, Charrier Lucie, Nicol Lionel, Kowala Mark, Ozoux Marie-laure, Lawson Francesca, Janiak Philip, Mulder Paul